
Anais da Academia Brasileira de Ciências (2019) 91(4): e20190310
(Annals of the Brazilian Academy of Sciences)
Printed version ISSN 0001-3765 / Online version ISSN 1678-2690
http://dx.doi.org/10.1590/0001-3765201920190310
www.scielo.br/aabc | www.fb.com/aabcjournal
An Acad Bras Cienc (2019) 91(4)
BIOLOGICAL SCIENCES
In vitro generation of human monocyte-derived dendritic cells
methodological aspects in a comprehensive review
GIOVANA CECHIM and JOSÉ A.B. CHIES
Laboratório de Imunogenética, Departamento de Genética, Instituto de Biociências, Universidade Federal do Rio Grande
do Sul/UFRGS, Av. Bento Gonçalves, 9500, Prédio 43323, Campus do Vale, 90501-930 Porto Alegre, RS, Brazil
Manuscript received on March 15, 2019; accepted for publication on June 1, 2019
How to cite: CECHIM G AND CHIES JAB. 2019. In vitro generation of human monocyte-derived dendritic
cells methodological aspects in a comprehensive review. An Acad Bras Cienc 91: e20190310. DOI 10.1590/0001-
3765201920190310.
Abstract: Dendritic cells (DCs) initiate and shape both innate and adaptive immune responses. They are
specialized in antigen presentation to naive T cells, thereby orchestrating the T cell immune responses.
Human peripheral blood and tissues contain several subsets of phenotypically and functionally distinct
DCs, which promote interactions between the external environment and lymphoid organs. Because of the
diculty in purifying these cells, in vitro studies only became more frequent when Frederica Sallusto and
Antonio Lanzavecchia developed a method to generate DCs from blood monocytes in vitro. Nowadays a
wide range of biotechnological innovations has allowed the study of DCs and their precursors in the most
diverse situations faced by the immune system. As a result of such studies, monocyte-derived dendritic
cells (MDDC) are presently used in clinical protocols for the treatment of a variety of diseases, including
cancer and human immunodeciency virus infection. We summarize recent advances in the understanding
of methodologies and inputs used in protocols to dierentiate DCs from blood monocytes in vitro.
Key words: Cytokines, dendritic cell, differentiation, monocytes.
Correspondence to: José Artur Bogo Chies
E-mail: [email protected]
ORCid:https://orcid.org/0000-0001-7025-0660
INTRODUCTION
In 1973, Ralph M. Steinman and Zanvil A. Cohn
described a new cell type that had a cytoplasm with
pseudopodia structures of various sizes and shapes,
giving the cell a starry aspect, much like neuronal
dendrites (Steinman and Cohn 1973). This work
described the cell currently known as a Dendritic
cell (DC). In 2011, the importance of this discovery
was recognized with the Nobel Prize in Physiology
or Medicine (Steinman 2012). However, the study
of these cells only had significant advances in
the 1990’s, when Frederica Sallusto and Antonio
Lanzavecchia rst developed a method allowing
the generation of DCs from blood monocytes. In
this study, monocytes obtained from peripheral
blood were differentiated into myeloid DCs in
the presence of granulocyte-macrophage colony
stimulating factor (GM-CSF) and interleukin (IL)-
4, (Sallusto and Lanzavecchia 1994).
DCs are the major antigen presenting cells
(APCs) due to their unique ability to activate naive
T cells (Steinman and Witmer 1978, Banchereau
et al. 2000). DCs are bone marrow derived,
originating from both myeloid and lymphoid
precursors and can encompass a range of cell types
with several phenotypes and functions (Ardavin et
al. 1993, Banchereau et al. 2000, Ueno et al. 2007,
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 2 | 21
Merad et al. 2013). They are distributed throughout
the body but are especially present in regions such
as the skin and mucous membranes, promoting
interactions between the external environment and
lymphoid organs (Steinman 1991, Guermonprez
et al. 2002, Granot et al. 2017, Worbs et al. 2017).
Due to its migratory patterns, DCs are called “the
Sentinels” of the immune system (Stockwin et al.
2000, Randolph et al. 2008, Merad et al. 2013,
Worbs et al. 2017).
Generally, these cells are found inside tissues
in a so-called “immature” state characterized by
a high capacity to capture and process antigens
and a limited capacity to stimulate T cells. In the
immature state, DCs exhibit low expression of the
co-stimulatory molecules CD80 and CD86, as well
as a low expression of major histocompatibility
complex (MHC) class II molecules. The presence
of “danger signals” triggers morphological changes
in DCs, inducing a process known as maturation,
which is characterized by a reduction of the DC
endocytic capacity and the increased expression
of MHC class II and co-stimulatory molecules
(Banchereau et al. 2000, Jore et al. 2009, Dalod et
al. 2014, Worbs et al. 2017).
Considering this cell type diversity, DCs may
be classied according to several criteria, including
their location, such as skin, lungs, and lymphoid
organs, since their function is intimately linked to
the compartment where they are located (Wu and
Liu 2007). Within this anatomical classication,
it is also possible to separate DCs in two further
categories: the migratory DCs, which migrate
continuously from peripheral tissues to the
draining lymph nodes via the aerent lymphatic
vessels, and resident DCs, which are found in the
tissues, and when activated, migrate to the draining
lymph nodes (Randolph et al. 2008, Segura et
al. 2012, Merad et al. 2013, Pulendran 2015). In
this regard, DCs are usually classied according
to their different abilities to drive the immune
response by dierential antigen presentation or by
modulating the immune system through cytokine
secretion (Jore et al. 2009, 2012, Kurts et al. 2010,
Pulendran 2015). Nowadays DCs are evaluated
according to their transcriptional proles in order
to understand the regulation of the development
and dierentiation of the distinct DC lineages (Belz
and Nutt 2012, Hammer and Ma 2013, Merad et al.
2013, Collin and Bigley 2018).
Due to its central role in immune responses,
a better understanding of the physiology and
function of the DCs will help us to improve the
use in clinical protocols to treat several diseases
such as cancer and human immunodeciency virus
(HIV) infection (Banchereau et al. 2001, Turville et
al. 2002, Kavanagh and Bhardwaj 2002, Gandhi et
al. 2016, Garg et al. 2017).
This review presents key information
about the generation of human DC from blood
monocytes (MDDC). We will analyze alternatives
for the purification of precursor cells (meaning
the monocytes), the selection of suitable culture
medium, appropriate culture medium supplements,
growth factors, and cytokines. We will also review
the most common molecular markers used to
characterize DCs by ow cytometry. The Figure
1 presents a schematic summary of the steps in a
monocyte-derived dendritic cell culture.
SOURCES OF MONOCYTES
The rst step for monocyte-derived dendritic cell
culture is to choose the monocyte source, since
these cells are the preferred precursors for in vitro
MDDC generation. One possibility is to directly
collect blood by venipuncture. The advantage of
whole blood as a monocyte source is the freshness
of the material. However, the disadvantage of using
whole blood is the low yield of monocytes, since
they represent only 6% of all peripheral blood cells,
so using whole blood requires the processing of
a large blood volume (Meyer et al. 2005, WHO
2010, Gillio-Meina et al. 2013).
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 3 | 21
The use of the cells, which are normally
discarded after the processing of donated blood,
is a way to skip such a problem. Commonly, after
blood donation, the blood bag is centrifuged,
which allows the separation of its components into
erythrocytes, plasma, and a bu y coat, which are
collected into separate bags. The bu y coat is a
concentrate of platelets and leukocytes which
can be further processed for platelet puri cation.
The remaining material is rich in leukocytes and
constitutes an excellent source of monocytes, but is
commonly discarded. Processing a 450 mL blood
bag usually generates 30–80 mL of bu y coat with
approximately 1x10
9
cells (Ito and Shinomiya 2001,
Repnik et al. 2003, Meyer et al. 2005, Strasser and
Eckstein 2010).
When plasmapheresis is performed in order to
obtain distinct blood products, leukocytes are often
collected together with platelets as a byproduct and
must be removed to avoid immune rejection in the
recipient. Therefore, leukocytes are ltered out by
leukoreduction and collected into leukoreduction
system chambers (LRSC) or leukocyte removal
filters also known as buffy cones (Ebner et al.
2001, Dietz et al. 2006, Strasser et al. 2007). The
bu y cone usually contains 10 mL of a processed
mixture with approximately 1x10
9
cells.
Another possible source for large quantities
of leukocytes is a leukopak. This is an enriched
leukapheresis product consisting of a variety of
blood cells including monocytes, lymphocytes, and
erythrocytes. There are two types of leukopaks:
one is collected from peripheral blood without
any stimulation on the blood donor and the other
are obtained from donors who were stimulated
with G-CSF (granulocyte colony stimulating
factor) to induce leukocyte production and trigger
migration of stem cells from bone marrow into
the bloodstream. Usually, leukopaks are collected
from healthy donors, but for research purposes it
is possible to obtain leukopaks from donors that
present certain pathologies such as hematological
malignancies and diabetes. Although the production
of leukopaks from such speci c donors is not usual,
this kind of product can be commercially provided
under request. Commercial leukopaks generally
contain 80–200 mL of processed material with
approximately 7x10
9
peripheral blood mononuclear
cells (PBMC), (more information available on:
www.allcells.com/products/whole-tissue/leuko-
pak). The Table I shows the percentage of cell
types and the amount of cells found in di erent
monocyte sources.
MONOCYTE PURIFICATION
After choosing the source of cells, the next
step in MDDC culture is to process the sample
Source
selection
•Peripheral blood
•Leukoreduction system chambers
•Buffy coat
•Leukopacks
Monocyte
purification
•Density gradient centrifugation
•Adherence to inert surface
•Magnetic-beads/antibody based cell isolation
•Fluorescent antibody based cell sorting
Monocyte-derived
dendritic cell
culture
•Cytokines
•Culture medium
•Supplements of the culture medium
•Culture time
•In vivo activation of immature dendritic cell
Cytometry
characterization
•Immunophenotype markers
Figure 1 - Scheme of the steps of a MDDC culture.
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 4 | 21
(Elkord et al. 2005). It is possible to use density
gradient media to separate blood components.
One of the most commonly used density gradient
medium is Ficoll
®
Paque Plus (GE Healthcare
Life Sciences – Catalog number: 17144003).
Ficoll
®
Paque Plus is a synthetic sucrose polymer
that ensures the separation of blood components
as follows: erythrocytes and polymorphonuclear
cells (eosinophils and neutrophils) are denser than
Ficoll
®
Paque Plus and therefore are deposited on
the bottom of the tube. Immediately above the
erythrocytes a density gradient medium Ficoll
®
Paque Plus layer forms (density of 1.077 G/mL
at 20°C). Above the Ficoll
®
Paque Plus layer,
PBMCs form a layer of cells similar to a cloud,
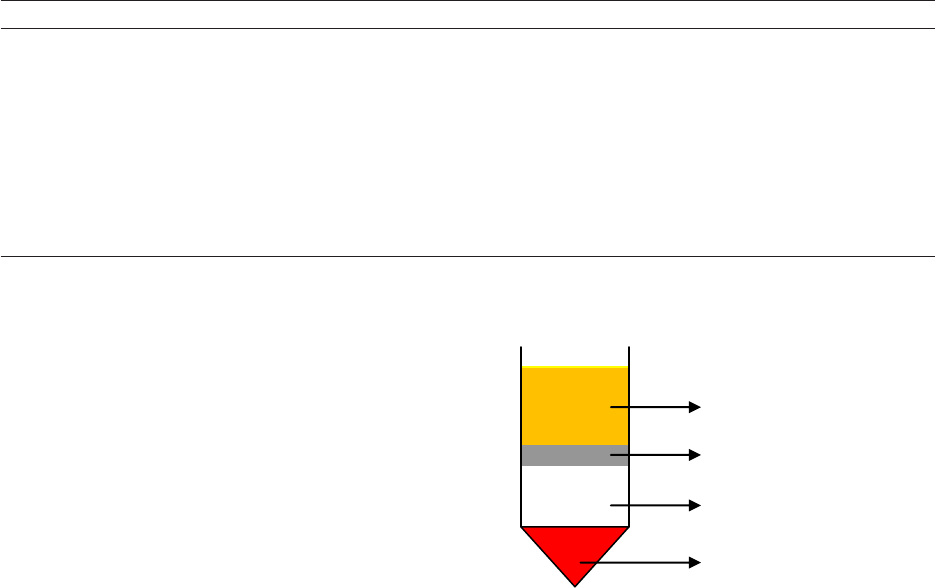
while the plasma is the uppermost layer in the tube
(see Figure 2). The PBMC layer includes B and
T lymphocytes, monocytes, NK (Natural Killer)
cells, and dendritic cells (Meyer et al. 2005).
This methodology is highly ecient and recovers
around 95% of the mononuclear cells present in the
original blood sample (Ito and Shinomiya 2001,
Lehner and Holter 2002).
Since monocytes are the only circulating
blood cells to show high expression of CD14 on
their membrane, this molecule is widely used as a
biomarker for monocytes and as a target for their
purication (Patel et al. 2017). Another distinct
characteristic of monocytes is the ability to adhere
to inert surfaces like plastic, dierent from other
cells present in the PBMC fraction (Patarroyo
et al. 1988). Protocols that take advantage of
this characteristic usually seed PBMC cells in a
plastic ask with the appropriate culture medium
(presented in the “Culture Medium” topic) and
allow adherence for 2 hours in a humidified
incubator. All monocytes will adhere to the culture
ask while B and T lymphocytes, NK cells, and DCs
will remain non-adherent and can be eliminated as
oating cells.
Another system to isolate monocytes is
the use of magnetic beads coated with specific
antibodies. Such magnetic bead-based cell
isolation methodology can be performed using both
a positive or a negative selection approach. In a
negative selection approach using the Dynabeads™
TABLE I
Percentage of cells in whole blood and blood products in health individuals.
Whole blood (%) Buy coat (%) LRSC (%) Leukopak (%)
CD4
+
T cells 22.5 24.4 32.39 31,92
CD8
+
T cells 6.8 10.8 15.04 17.13
B cells 5.2 7.2 11.33 12.52
Monocytes 8.4 10.7 20.28 19.08
NK cells 4.4 4.1 10.21 7.65
Eosinophils 3.2 1.3 1.88 1.07
Neutrophils 53.8 34.4 2.89 3.91
PBMC 2x10
6
cells/mL 1x10
9
cells 1x10
9
cells 7x10
9
cells/half
LRSC: Leukoreduction System Chamber. NK: Natural Killer. PBMC: Peripheral Blood Mononuclear Cells. Modied from: https://
www.miltenyibiotec.com/US-en/resources/macs-handbook/human-cells-and-organs/human-cell-sources/blood-human.html.
Plasma
Ficoll® Paque Plus
Red blood cells
Mononuclear layer
Figure 2 - Schematic diagram of the separation of the
components of a blood sample from Ficoll
®
Paque Plus
density gradient medium.
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 5 | 21
Untouched™ Human Monocytes Kit, for example,
(Invitrogen, Norway, Catalog number: 11350D),
a mixture of biotinylated monoclonal antibodies,
each one of them specic against a distinct surface
cell marker [anti-CD3 (a typical T lymphocyte
antigen), anti-CD7 (a typical T- and NK-
lymphocyte antigen), anti-CD16a and anti-CD16b
(typical antigens expressed in T lymphocytes, DCs,
NK cells, macrophages and granulocytes), anti-
CD19 (expressed in B lymphocytes), anti-CD56 (a
NK cell surface molecule), anti-CDw123 (a typical
plasmacytoid DCs antigen) and anti-CD235a (a
typical antigen expressed in erythrocytes and stem
cell precursors)] is added to the sample. After that,
beads coupled with streptavidin are added. Of note,
these beads act as superparamagnetic particles,
meaning that they exhibit magnetic properties when
placed in a magnetic eld. After a short incubation
period, the streptavidin-beads will bind to the
biotinylated-antibody-labeled cells. A magnet is
applied to the sample and the cells linked to the
biotinylated-antibody-streptavidin-beads will be
attracted and will be immobilized by the magnet
whereas cells not linked to any of these antibodies
will be free in the supernatant and can be washed
out and collected. Since the mixture of antibodies
encompasses specicities against all PBMCs, but
not against monocytes, this system will capture
all non-monocyte cells. Importantly, no binding
will occur between antibodies and monocytes, and
therefore the isolated cells will be recovered after
a relatively mild handling, without any activation
mediated by antibodies.
Conversely, using a positive selection
approach with anti-human CD14 MicroBeads from
Miltenyi Biotec, Germany, (Catalog number: 130-
050-201), for example, the cell sample is incubated
with an anti-CD14 antibody bound to a magnetic
bead. During this incubation period, the anti-CD14
antibody binds to the CD14-positive cells present
in the sample and the cell suspension is loaded onto
a column which contains a magnet that induces a
high-gradient magnetic eld. Thus, the magnetically
labeled CD14-positive cells are retained within the
column and the unlabeled cells run through. After
removing the column from the magnetic field,
the magnetically retained CD14-positive cells are
eluted. It is worthy of note that the CD14 molecule
belongs to the lipopolysaccharide (LPS) receptor
complex. Recognition through this receptor is
interpreted by the cell as a “danger signal” (Jore et
al. 2009) capable of inducing a maturation process
on immature DCs (more details in “Activation of
Immature Dendritic Cell”). However, according
to the manufacturer, the binding of an antibody to
CD14 does not trigger signal transduction since
CD14 alone lacks a cytoplasmic domain. (Akira
and Takeda 2004, Liew et al. 2005, Napolitani et al.
2005). The advantages of using magnetic selection
are high purity (>95%) and speed (Delirezh et al.
2013).
Another technical alternative for cell
separation involves ow cytometry. Fluorescent
antibody-based cell sorting is a specialized type of
ow cytometry used in the isolation of a specic
cell type from a heterogeneous mixture of cells.
The use of specic antibodies labeled with distinct
uorochromes allied to data about cell size and
complexity allows the sorting of cells based on the
presence or absence of specic molecules on its
surface. Moreover, this technique is the only one
capable of distinguishing and separating cells with
dierent levels of expression of a given molecule
within a population. Thus, through ow cytometry
it is possible, for example, to isolate CD14
high
CD16
-
cells from a complex sample like total PBMCs, in
a single procedure. As a consequence, cells will
be subjected to less handling and, therefore, will
be exposed to less stress. In this sense, when
the objective is the isolation of a cell population
dened by multiple simultaneous features (meaning
the presence/absence of several surface markers
in distinct expression intensities) cell sorter ow
cytometry is the technique of choice. However,
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 6 | 21
drawbacks of this technique include the high cost
of both the equipment and the accessories needed
to ensure operator biosafety due to aerosols formed
during sorting (Holmes et al. 2014).
CYTOKINES AND DCs GENERAL FEATURES
Sallusto and Lanzavecchia (Sallusto and
Lanzavecchia 1994) rst achieved in vitro MDDC
generation through medium supplementation
with distinct cytokines, in this case, through
the combined use of IL-4 and GM-CSF. In the
following years, several works characterized
dierent protocols concerning the use of distinct
cytokines in order to induce and support in vitro
monocyte differentiation and maturation in
MDDCs. It is of crucial importance to note that
dierent combinations of cytokines will generate
MDDCs with distinct characteristics and functions,
so we should choose the cytokines that best match
the purpose of the work. The cytokines most
frequently used for in vitro MDDC dierentiation
and the main characteristics of the generated DCs
will be briey reviewed in the next paragraphs.
Sallusto and Lanzavecchia used the combination
of IL-4 and GM-CSF to promote the dierentiation
of monocytes into DCs. GM-CSF growth factor
seems to down-regulate the expression of the
macrophage colony-stimulating factor (M-CSF)
receptor on monocytes, thereby inhibiting
M-CSF induced dierentiation of monocytes into
macrophages (Suzuki et al. 2004). Similarly, IL-4
exerts its actions in monocytes dierentiation by
inhibiting macrophage colony formation (Jansen et
al. 1989, Relloso et al. 2002). Reports also suggest
that IL-4 can activate some properties of monocytes
and up-regulate MHC class-II molecules, Dendritic
Cell-Specific Intercellular adhesion molecule-
3-Grabbing Non-integrin (DC-SIGN) and co-
stimulatory molecules, and down-regulate CD14
(Ruppert et al. 1991, Ulanova et al. 2001, Relloso
et al. 2002, Sander et al. 2017). The DC generated
by this protocol, after seven days in culture, show
a typical dendritic morphology. The phenotype
of this DC is characterized by high membrane
expression of MHC class I and class II molecules,
CD1a, CD1b, CD1c, FcγRII, ICAM-1, CD11b,
CD11c, CD40, B7, and CD33. In this work, DCs
were also positive for Ii, LFA-1, LFA-3, and CD44.
The expression of CD14 was either low or negative
and these DCs were also negative for FcγRI and
FcγRIII. They also presented a high capacity to
stimulate allogeneic and autologous T cells and a
unique ability to stimulate naive T lymphocytes,
being efficient at presenting soluble antigens
(tetanus toxoid) to a specic T cell (Sallusto and
Lanzavecchia 1994).
Sanarico and colleagues (Sanarico et al. 2006),
described another methodology for inducing
dierentiation of MDDCs in vitro using GM-CSF,
IL-4, and IL-2. The DCs described by this group
show the same morphology and phenotype of the
DCs generated in the presence of GM-CSF and IL-
4. After LPS stimulation, it was observed that there
was an up-regulation of activation markers such as
human leukocyte antigen (HLA)-ABC, HLA-DR,
CD80, CD86, and CD83 but not CD25 (the IL-2
receptor subunit). However, some dierences were
observed regarding the DCs generated with GM-
CSF and IL-4 only, such as a signicantly higher
secretion of IL-1β, Tumor Necrosis Factor (TNF)
-α, and IL-12p70 in response to LPS stimulation.
They also demonstrated the capacity to induce high
interferon (IFN) γ secretion by allogeneic naive T
cells (Sanarico et al. 2006).
Geissmann and coworkers developed a
protocol for generating Langerhans cells (LC) in
vitro, which are specic DCs found on the human
skin (Perussia et al. 1985, Geissmann et al. 1998).
They added GM-CSF, IL-4, and transforming
growth factor (TGF) β to monocyte culture.
The predominant TGF-β isoform expressed
in the immune system, TGF-β1, regulates cell
differentiation and survival during Langerhans
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 7 | 21
cell development (Bauer et al. 2012, Esebanmen
and Langridge 2017). TGF-β also induces
immature DCs to present an immunosuppressive
phenotype thereby ensuring system homeostasis by
downregulating the function of these cells (Li et al.
2006, Travis and Sheppard 2014, Esebanmen and
Langridge 2017). The Langerhans cells generated
by this protocol, in an immature state, exhibit
Birbeck granules and express CD1a, E-cadherin,
and CLA, but do not express CD83 and CD86.
However, the addition of TNF-α and IL-1 induces
HLA class II, CD83, and CD86 expression and the
loss of E-cadherin expression. These cells are also
highly ecient in stimulating the proliferation of
allogenous lymphocytes (Geissmann et al. 1998).
A dierent combination of cytokines was used
to produce MDDC by Takahashi et al. (Takahashi
et al. 1997). A combination of GM-CSF and
IL-7 gave rise to floating cells with typical DC
morphology and some adherent cells developed the
appearance of Langerhans cell-like dendrites. The
typical DC presents a membrane phenotype with
high expression of MHC class I and class II, CD1a,
CD11c, CD23, CD40, CD54, CD58, CD80, CD86,
and CD95, and decreased CD14 expression (very
low or absent). DCs induced by this protocol are
also positive for CD21, weakly positive for CD32,
and negative for CD16. Compared to PBMCs, the
DCs generated by this protocol, also called G7 DC
by the authors, are more eective in stimulating
autologous and allogeneic T cell proliferation.
G7 DCs are more eective in inducing peptide-
specic CTL activity than the DCs generated with
GM-CSF and IL-4 (Takahashi et al. 1997). The role
of IL-7 in monocyte dierentiation in DCs is not
fully elucidated. Studies on IL-7Rα
-/-
and IL-7
-/-
mice demonstrated that IL-7 may play an important
role in the development of DCs and plasmacytoid
dendritic cells (pDCs) but further investigations are
required (Yang et al. 2005, Vogt et al. 2009).
Two different groups used the combination
of GM-CSF and IFN-α to generate MDDC in
vitro (Santini et al. 2000, Mohty et al. 2003). In
both cases, the DCs generated with GM-CSF and
IFN-α exhibited a typical DC morphology and
show an immature phenotype which demonstrates,
after exposure to maturation stimulus (CD40L or
LPS), up-regulated expression of co-stimulatory
molecules CD40, CD80, CD86, and high
expression of CD83, HLA-DR, and MHC class
I. The prominent ability of these DCs to secrete
IFN-α suggests an ability to promote a Th1
response. Mohty and colleagues also measured the
expression of CD1a, blood dendritic cell antigen
(BDCA) -4, CD54, CD58, and DC lysosome-
associated membrane protein (DC-LAMP) on
MDDC, which showed an increase in expression
after the maturation stimulus with CD40L for 2
days. The DCs generated by Mohty and colleagues
(called as IFN-DCs by the authors) dierentially
secreted, depending on their maturation stage, large
amounts of inammatory cytokines such as IL-1β,
IL-6, IL-10, TNF-α, and especially IL-18 (which
could be detected at both maturational stages).
However, these cells did not secrete IL-12p70.
In the immature state, IFN-DCs induced a potent
autologous antigen-specic immune response. Like
the natural type I IFN-producing plasmacytoid
DCs, the IFN-DCs expressed a several Toll-like
receptors (TLR) including TLR7 and could secrete
IFN-α following viral stimulation or TLR7-specic
stimulation (Ito et al. 2002, Mohty et al. 2003, Liu
2005).
Santini and coworkers observed that, in
response to type I IFN (IFN-α, IFN-α2b and IFN-
β) and GM-CSF treatment, adherent monocytes
became non-adherent cells within three days of
culture. The loss of adherence was associated
with cellular aggregation in large cell clusters.
However, a considerable percentage of the cells
underwent apoptosis after ve days in culture, and
it is important to note that TNF-related apoptosis-
inducing ligand (TRAIL) and TRAIL receptors
(R1 and R2) were upregulated in response to
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 8 | 21
LPS stimulus (Wang and El-Deiry 2003). The
DCs obtained by Santini and coworkers showed a
stronger capability to stimulate the proliferation of
allogeneic lymphocytes than DCs generated with
GM-CSF and IL-4. In particular, DCs generated in
the presence of type I IFN and GM-CSF showed
a potent ability to take up, process, and present
inactivated HIV-1 to autologous T lymphocytes in
vitro when compared to DCs generated with GM-
CSF and IL-4 (Santini et al. 2000, Biron 2001, Ito
et al. 2001).
IL-3 cytokines are also used in association
with IFN-β or IL-4 in protocols to produce MDDC.
(Buelens et al. 2002, Ebner et al. 2002). DCs
generated by these protocols acquire a dendritic
morphology and show similarities in phenotype.
The expression of CD14 molecules is low or absent,
but high levels of BDCA-4, and CD11c are found
when compared to DCs generated with GM-CSF
and IL-4. Only CD1a was dierentially expressed,
either at low levels or not at all. After a maturation
stimulus, DCs upregulated the costimulatory
molecules CD40, CD80, CD86, CD83, DC-LAMP,
HLA-DR, and MHC class I (Biron 2001, Buelens
et al. 2002, Ebner et al. 2002).
The IL-15 cytokine is used in two
different protocols to generate MDDC in vitro
(Mohamadzadeh et al. 2001, Saikh et al. 2001).
Saikh and colleagues use only IL-15 to promote
monocyte differentiation into DC. The DCs
generated by Saikh and colleagues show surface
levels of the costimulatory molecules CD86,
CD80, CD40, and CD83 that were equivalent to
DC obtained from cultures of monocytes treated
with GM-CSF plus IL-4 and stimulated with
TNF-α. However, the DCs generated with IL-15
did not express CD1a. Another important feature
of Saikh’s DCs is the ability to stimulate strong
allo-responses and produce signicant amounts of
IFN-γ and IL-12 when compared to DCs generated
with GM-CSF plus IL-4 and stimulated with TNF-α
(Saikh et al. 2001, Dubois et al. 2002, 2005).
Mohamadzadeh’s group differentiated
monocytes in the presence of IL-15 plus GM-CSF
(Mohamadzadeh et al. 2001). The resulting cells,
after six days in culture, had a phenotype positive
for CD1a molecule, negative for CD14, and an
increased in surface expression of HLA-DR,
CD40, CD80, CD86 and CD83. The intracellular
expression of DC-LAMP was observed upon LPS
activation. The interesting features about this DC
are the expression of Langerhans cell markers such
as E-Cadherin, Langerin, and chemokine receptor
(CCR) 6. Nevertheless, they do not express
Birbeck granules like a “genuine” Langerhans cell
(Mohamadzadeh et al. 2001, Dubois et al. 2002,
2005).
TNF-α is used together with GM-CSF by
Iwamoto and coworkers to induce the dierentiation
of monocytes into DCs (called TNF-DC by the
authors), (Iwamoto et al. 2007). The culture of
CD14
+
monocytes was incubated for seven days
in the presence of GM-CSF and TNF-α, inducing
a spindle-shaped and adherent morphology. Two
days after LPS stimulation, they began to convert
to DC-like oating cells with extended dendrites.
The TNF-DC expressed low levels of CD14 and
substantial levels of HLA-DR, CD40, CD70, CD80,
CD86 and CD83, and produced extremely large
amounts of TNF (>150 ng/mL at peak). They also
produce IL-12/IL-23p40 and IL-23p19/p40, but
very little IL-12p70 compared to DC generated with
IL-4 and GM-CSF. The TNF-DC had the capacity
to induce naive CD4 T cells to produce IFN-γ and
TNF-α and stimulated resting CD4 T cells to secret
IL-17 when compared to DC generated with IL-4
and GM-CSF (Chomarat et al. 2003, Wajant et al.
2003, Iwamoto et al. 2007, Brenner et al. 2015).
The Table II presents a summary of the cytokines,
growth factors and maturation inducing substances
used by the dierent groups described above.
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 9 | 21
TABLE II
Combination of cytokines, grown factors and maturation stimulus for MDDC culture in vitro.
Cytokines Concentration
Time to add
cytokines
Stimulus for maturation
Maturation stimulus
added
Stimulation
period
Total culture
time
References
GM-CSF +
IL-4
50 ng/mL
1
+
1000 U/mL
1
1
o
day
TNF-α (10 ng/mL)
2
CD40L
3
Not
mentioned
24 hours
24 hours
Seven days
Sallusto and
Lanzavecchia 1994
GM-CSF +
IL-7
600 U/mL
4
+
6 U/mL
5
1
o
day Not
mentioned
Not
mentioned
Not mentioned Seven days Takahashi et al. 1997
GM-CSF +
IL-4 +
TGF-β1
250 ng/mL
6
+
100 ng/mL
7
+
10 ng/mL
8
1
o
day
9
TNF-α
10
+
IL-1
10
Not
mentioned
Not mentioned Seven days
Geissmann et al. 1998
GM-CSF +
IFN-α
500 U/mL
11
+
1000 U/mL
12
1
o
day
LPS (1 µg/mL)
13
3
o
day 48 hours
Five
days
Santini et al. 2000
GM-CSF +
IFN-α
100 ng/mL
14
+
500 IU/mL
15
1
o
day
16
LPS (10 µg/mL)
17
or
Poly(I:C)(15µg/mL)
18
or
CD40L
19
5
o
day 48 hours Seven days Mohty et al. 2003
GM-CSF +
IL-15
100 ng/mL
20
200 ng/mL
21
1
o
day LPS (100 ng/mL)
22
Not
mentioned
Not mentioned
Six
days
Mohamadzadeh et al.
2001.
IL-15
100 ng/mL
23
1
o
day
Not
mentioned
Not
mentioned
Not
mentioned
Seven
days
Saikh et al. 2001
IL-3 +
IL-4
100 U/mL
24
1000 U/mL
25
1
o
day
TNF-α (10 ng/mL)
26
+
IL-1β (10 ng/mL)
27
+
IL-6 (1000U/mL)
28
+
PGE2 (1 µg/mL)
29
7
o
day 48 hours
Nine
days
Ebner et al. 2002.
IL-3 +
IFN- β
50 U/mL
30
1000 U/mL
31
1
o
day
LPS (1 µg/mL)
32
or
Inuenza virus
32
or
Poly[I:C](20 µg/mL)
33
CD40L
34
Not
mentioned
24 hours
24 hours
48 hours
3 days
Six
days
Buelens et al. 2002
GM-CSF +
IL-4 +
IL-2
50 ng/mL
35
10 ng/mL
35
100 U/mL
36
1
o
day
1
o
day
1
o
and 5
o
days
LPS (200 ng/mL)
37
5
o
day 48 hours Seven days Sanarico et al. 2006
GM-CSF +
TNF-α
10 ng/mL
38
10 ng/mL
38
1
o
day
LPS (10 µg/mL)
39
Not
mentioned
Several time
intervals
Seven days Iwamoto et al. 2007
1
Own lab production.
2
Reagent supplied by Dr. Manfred Brockhaus (Homann-La Roche, Basel, Switzerland).
3
A soluble chimeric fusion protein between the mouse CD8 α-chain and the human
CD40 ligand (CD40L) was a generous gift of Dr. Peter Lane (Basel Institute for Immunology).
4
Pepro Tech Inc. (Rocky Hill, NJ) or provided by Prof. Nicos Nicola (The Walter and Eliza Hall
Institute of Medical Research, Victoria, Australia).
5
Pepro Tech Inc.
6
Sandoz AG (Bale, Switzerland).
7
Genzyme Corp. (Cambridge, MA).
8
R&D Systems, (Minneapolis, MN).
9
At days 2 and 4, half
of the medium was removed and an equivalent volume of fresh medium, supplemented with GM-CSF, IL-4 andTGF-β1 was added.
10
Concentrations not mentioned.
11
R&D Systems.
12
CIFN is a
synthetic type I IFN produced from recombinant DNA, whose sequence is based on a consensus derived from the amino acid sequences of the most common types of human IFN- α. (CIFN, specic
activity of 10
9
U/mG protein), Amgen.
13
Sigma-Aldrich.
14
Leucomax, Novartis (Rueil-Malmaison, France).
15
Introna, Schering-Plough (Levallois-Perret, France).
16
The medium was replenished
with cytokines every 3 days.
17
LPS from Escherichia coli, serotype O26: B6, Sigma-Aldrich (St. Quentin Fallavier, France).
18
Polyriboinosinic polyribocytidylic acid- poly(I:C), (Sigma-Aldrich).
19
Murine L cells transfected with human CD40L were kindly provided by Schering-Plough (Laboratory for Immunological Research, Dardilly, France), used after 75 Gy irradiation (ratio, 1:10).
20
Leukine, Immunex.
21
(R&D Systems or Schering-Plough).
22
Sigma-Aldrich.
23
PeproTech Inc (Rocky Hill, NJ).
24
PeproTech (London, U.K.; sp. act., 10
7
U/mG).
25
Genzyme (Cambridge, MA; sp.
act., 5.10
7
U/mG).
26
TNF-α (sp. act., 6X10
7
U/mG) was provided by Dr. G. R. Adolf (Bender, Vienna, Austria).
27
IL-1β (sp. act., 5X10
8
U/mG), Genzyme.
28
IL-6 (sp. act., 1X10
7
U/mG), Genzyme.
29
PGE2 (prostine E), Pharmacia & Upjohn (Buurs, Belgium).
30
R&D Systems Europe (Oxon, United Kingdom).
31
Ares Serono Europe (London, United Kingdom).
32
Provided by N. Kuehm,
Aventis, Pasteur Merieux (Val de Reuil, France).
33
Sigma.
34
DCs were activated by coculture with irradiated 3T6 broblasts transfected with the human CD40L gene (CD40L transfectants) (5x10
4
/
mL).
35
EuroClone.
36
R&D Systems (Minneapolis, MN).
37
Sigma-Aldrich (St. Louis, MO).
38
Diaclone Research.
39
LPS from Escherichia coli (L4516), Sigma-Aldrich.
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 10 | 21
CULTURE MEDIUM
The importance of the culture medium in the in
vitro induction of DCs is undeniable since it should
provide a suitable environment (an appropriate pH
and all the necessary nutrients and factors) for cell
maintenance, growth, and dierentiation. There are
many dierent culture media described and used
in the extensive literature on MDDC. To make the
best choice, it is necessary to consider the general
and specic objectives of the experiments, as well
to take into consideration the cost of the dierent
supplements to be used (Duperrier et al. 2000, Peng
et al. 2005, Janetzki et al. 2010).
If the main objective encompasses the
clinical (therapeutic grade) use of the MDDCs
generated in vitro, such as vaccines, the only
Food and Drug Administration (FDA) approved
culture medium is the AIM-V (GIBCO, Catalog
number: 087-0112DK). The complete AIM-V
composition is not informed by the manufacturer
(GIBCO), although the manufacturer provides
some important information (in this sense, it is
reported that this medium does not contain serum
but contains L-glutamine and antibiotics - 50 µg/
mL streptomycin sulfate and 10 µg/mL gentamicin
sulfate).
For research purposes, PromoCell, for
example, oers three dierent medium types: DC
Generation Medium (Catalog number: C-28050),
DC Generation MediumDXF (Catalog number:
C-28052), and Monocyte Attachment Medium
(Catalog number: C-28051). As in the previous
example, the complete composition is not disclosed.
The only information available states that the DC
Generation Medium DXF is free from animal-
derived components, although it contains human
serum albumin. An important point, according to
the manufacturer, is that the Monocyte Attachment
Medium allows an ecient selection of monocytes
from freshly isolated mononuclear cells through
differential adherence and immunomagnetic
purication steps. For all media, supplementation
with growth factors and cytokines is needed
(Challagundla et al. 2015).
Another culture medium used to generate
MDDC for research purposes is X-VIVO 15.
The complete composition is not reported by the
manufacturer (LONZA), so the only information
available is that it is chemically dened and serum-
free. There are versions with or without L-glutamine,
gentamicin, recombinant transferrin and phenol red.
As previously stated, all media requires cytokine
and growth factor supplementation, depending on
the specic goal of the experiment and the initial
cell source.
A commonly used medium in cell culture
experiments is RPMI-1640. It was developed
by Moore and colleagues at the Roswell Park
Memorial Institute, hence the acronym RPMI
(Moore et al. 1967). The exact RPMI-1640
composition is available and well established,
which allows the production of quite similar
media by several manufacturers. There are several
modied versions of RPMI, some of them lacking
specic components, others already supplemented
with certain growth factors or other molecules.
Nevertheless, as a general rule, supplementation
with serum, cytokines, and/or growth factors is
required.
SUPPLEMENTS FOR THE CULTURE MEDIUM
The widely used culture medium RPMI-1640
is also the only commercial medium for which
the complete formula is available. This enables
each researcher to direct the supplementation
specically according to the particularities of each
experiment. The most commonly used culture
medium supplements (biological or chemical
factors) for in vitro production of MDDCs will be
briey presented below.
An essential function of culture medium
consists in providing cells an environment with a
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 11 | 21
physiological pH, and thus, buering agents are
essential. Sodium bicarbonate (NaHCO
3
) is a
commonly used pH regulating agent, since it can
be used in cell culture without increased toxicity
to the cells. Sodium bicarbonate chemically reacts
either as acid or as a base due to its amphoteric
characteristic. In solution, sodium bicarbonate
produces bicarbonate ions, while the metabolism
of cells in culture produces carbon dioxide (CO
2
),
decreasing the culture medium pH (if the culture
medium contains phenol red, the color of the
medium will become yellowish). In addition,
the incubator also injects carbon dioxide into its
atmosphere, thereby increasing the carbon dioxide
concentration in the microenvironment and lowering
the culture medium pH. Thus, within the incubator
microenvironment, a sodium bicarbonate-carbon
dioxide buer balance is established. The carbon
dioxide dissolved in the culture medium, either
from the incubator or from the cell metabolism,
equilibrates with the bicarbonate ions, buering
the medium. The sodium bicarbonate concentration
in the medium should be in equilibrium with the
carbon dioxide level in the incubator atmosphere.
Thus, for media containing 1.5–2.2 g/L of sodium
bicarbonate, the incubator must inject 5% of
carbon dioxideinto its microenvironment. For
media containing 3.7 g/L of sodium bicarbonate,
the incubator should inject 10% of carbon dioxide.
If the sodium bicarbonate concentration is too high
in relation to the carbon dioxide level injected by
the incubator, the culture medium will alkalize, and
its color will turn pink (Barker 1998).
HEPES (4-(2-hydroxyethyl)-1-
piperazineethanesulfonic acid) is a buering agent
which is membrane impermeable, chemically
and enzymatically stable, demonstrates a limited
eect on biochemical reactions, and has very low
visible and UV light absorbance. HEPES provides
extra buering capacity when cell cultures require
extended periods of manipulation outside of a
CO
2
incubator. The HEPES concentration in cell
culture media may vary from 10 mM to 25 mM.
Although HEPES-buffered medium exposed to
uorescent light was shown to be cytotoxic, due
to the formation of free radicals, this effect is
preventable by manipulating the cells under light-
shielded conditions.
Serum is a quite ubiquitous supplement in
cell cultures. This component will supply mainly
growth factors and cytoines to the cells, although
generally it is not possible to dene the exact types
and concentrations of these components in the
serum. Many studies use human serum from an AB
serum pool to supplement cell cultures. The nal
concentrations of serum used vary from 1 - 10%.
It is also possible to use plasma or even umbilical
cord blood serum. Nevertheless, if the cells will
not be used in clinical applications, fetal bovine
serum is the least expensive choice, and is also
easier to obtain. However, it is crucial to observe
if the serum was inactivated. This inactivation is
necessary due to the presence of proteins from the
complement system which could cause cell death
(Anton et al. 1998).
Although cell culture procedures are
conducted in aseptic environments, any biological
contaminant can devastate the cell culture, and
therefore the use of antibiotics and antimycotics is
sometimes required. The most common antibiotics
used in cell cultures are penicillin-streptomycin and
gentamicin. Penicillin-streptomycin acts on Gram-
positive and Gram-negative bacteria whereas
gentamicin is also active against mycoplasma. The
recommended concentration for use in cell culture
for Penicillin-streptomycin are 50 to 100 I.U./
mL penicillin and 50 to 100 μg/mL streptomycin
(Catalog number: ATCC® 30-2300), and 5 to 50
μg/mL gentamicin (Barker 1998). In culturing cells
that are intended for clinical use, the FDA does not
recommend the use of penicillin or β-lactams due to
the possibility of severe hypersensitivity reactions
(Vatsan et al. 2013).
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 12 | 21
A widely used antifungal agent is amphotericin
B. It acts against both fungi and yeasts. The
recommended concentration for use in cell culture
is 0.25-2.5 μg/mL (GIBCO, Catalog number:
15290026).
A component often added to culture medium is
the amino acid glutamine. This amino acid can lead
to citrate production, which is exported to the DC’s
cytoplasm and acts as a substrate for fatty acids
synthesis, an essential process for the induction
of DC activation by TLR ligands. Due to its rapid
degradation and instability in solution, glutamine
supplementation must be performed after careful
consideration. The recommended concentration for
use in cell culture generally falls in the range of 2-6
mM (GIBCO, Catalog number: 21051024).
Free radicals are a natural side product of
the increased metabolism in monocytes and
in immature DCs during differentiation and
maturation. To avoid the toxic effects of the
accumulation of these reactive oxygen species in
the culture medium, 2-mercaptoethanol (2-ME)
is used as a reducing agent. Due to its instability
in solution, 2-mercaptoethanol should be added
daily. It is recommended to dilute 2-ME to 50 mM
in phosphate-buered saline (PBS), and add this
solution to the cell medium at a nal concentration
of 50 µM. (GIBCO, Catalog Number: 21985023),
(Click 2014).
CULTURE TIME
The time needed to establish a cell line or to induce
cell dierentiation in vitro is quite variable, and
several aspects should be taken into consideration.
The majority of studies consider that the
dierentiation process from monocyte to immature
DC is completed in ve days with an additional
period of 48 hours for maturation. However,
several works have been demonstrated that in
vitro development of mature DCs from monocyte
precursors does not require more than 2 days of
culture, dened by some as fast DCs (fDC), (Dauer
et al. 2003, Obermaier et al. 2003, Ramadan et al.
2004, Massa and Seliger 2013). The data about
phenotypic characteristics, immune properties, and
the pattern of responses induced for the fDC are
still poorly understood (Tanaka et al. 2006, Rojas-
Canales et al. 2012, Pavlović et al. 2015).
In vitro MATURATION OF IMMATURE
DENDRITIC CELL
In the majority of MDDCs generation protocols,
the resulting DCs are in an immature state. An
immature dendritic cell (iDC) shows a high
capacity to capture and process antigens but has
limited capacity to stimulate T cells due to the
low expression of the co-stimulatory molecules
CD40, CD80, CD86, and HLA-class II. The in
vitro maturation process is characterized by several
events and although dierent groups may dene
distinct features, the main events include the
downregulation of endocytic/phagocytic receptors,
the upregulation of costimulatory molecules CD40,
CD80 and CD86, the upregulation of CD58 and
CD83, the shift in lysosomal compartments with
downregulation of CD68, the upregulation of DC-
LAMP, and the changes in HLA-class II molecules
(Banchereau et al. 2000, Lutz and Schuler 2002,
Iwasaki and Medzhitov 2004).
Some important morphological changes
also occur during the DC in vitro maturation
process, including the loss of adhesive structures,
cytoskeleton reorganization, and the acquisition of
high cellular motility (see Table III). These changes
allow the DCs a better capacity for lymphocyte
stimulation (Banchereau et al. 2000, Dalod et al.
2014).
To induce the DC maturation process in vitro it
is necessary to give a stimulus that mimics a danger
signal (Matzinger 1994, Gallucci et al. 1999, Jore
et al. 2009, Castiello et al. 2011). It is important
to note that in a seven days protocol for MDDC
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 13 | 21
generation, the stimulus is usually given on day
5 and is maintained for 48 hours. In the case of
fDCs, the dierentiation cocktail already contains
substances that will induce cell maturation. The
main substances used to promote DC maturation in
MDDC cultures are described below (Haenssle et
al. 2008, Castiello et al. 2011, Li et al. 2012).
A common substance used to mimic DC
activation when provoked by bacteria is LPS,
a characteristic component of the wall of Gram-
negative bacteria (Raetz and Whiteld 2002). The
LPS molecule bis bound by the CD14 molecule
present on the cell surface and activates the TLR4/
MD-2 complex pathway, promoting the secretion
of pro-inammatory cytokines by DCs (Iwasaki
and Medzhitov 2004, Park and Lee 2013).
Nevertheless, due to its potential toxicity, LPS is
only used in research protocols. The majority of
protocols aiming for DC maturation use LPS in
a concentration range of 10 ng/mL to 10 µg/mL
(InvivoGen, Catalog code: tlrl-eklps).
Flagellin is another substance that mimics the
danger signal triggered by the presence of Gram-
negative and/or Gram-positive bacteria. Flagellin is
generally detected by TLR5 whereas intracellular
agellin is detected by NOD-like receptors (NLRs)
NLRC4 and NAIP5. The process of DC maturation
induced by agellin results in the activation of the
NF-κB signaling pathway and cytokine production
(Akira et al. 2001, Vicente-Suarez et al. 2009, Miao
and Warren 2010). It was already been suggested
that agellin could be used as a vaccine adjuvant
(Coman et al. 2010). As in the previous case of
LPS use, the agellin concentrations used in DC
maturation protocols widely vary, ranging from 10
ng/mL to 10 µg/mL (Vicente-Suarez et al. 2009),
(InvivoGen, Catalog code:tlrl-sta).
To mimic virus-mediated activation during
DC maturation it is possible to use commercially
available synthetic polynucleotide sequences such
as the double stranded RNA analog polyinosinic-
polycytidylic acid [poly(I:C)], the single-stranded
RNA analog poly-uridine (polyU), or the single-
stranded RNA analog guanosine- and uridine-rich
(GU-rich). Whereas poly(I:C) binds to TLR3, the
remaining two types of sequences bind to TLR7/
TLR8 (Heil et al. 2004, Schlee and Hartmann
2016). Alternatively, the use of compounds
derived from imidazoquinoline such as the analog
imiquimod can be used to activate immature DCs.
Imiquimod is a commercially available compound
which selectively binds to TLR7 (Xiao et al. 2016).
Poly(I:C) and imiquimod are also used as adjuvants
in protocols to develop cancer vaccines (Coman
et al. 2010), (InvivoGen, Catalog code: vac-pic).
Short synthetic single-stranded DNA molecules
that contain unmethylated CpG dinucleotides
(CpG) are also used as TLR9 agonists to activate
TABLE III
Characteristics of DCs according to dierent maturation stages. The characteristics described refers to the expression in
the plasma membrane.
Immature DC Mature DC
High intracellular expression of HLA-II High membrane expression of HLA-II
High endocytic/phagocytic capacity Low endocytic/phagocytic capacity
High membrane expression of CCR1, CCR5 and CCR6 Low membrane expression of CCR1, CCR5, and CCR6
Low membrane expression of CCR7 High membrane expression of CCR7
Low membrane expression of CD40, CD54 and CD58 High membrane expression of CD40, CD54, and CD58
Absent/low membrane expression of DC-LAMP High membrane expression of DC-LAMP
Low membrane expression of CD80, CD86 and CD83 High membrane expression of CD80, CD86, and CD83
High membrane expression of CD68 Low membrane expression of CD68
Induce T cell anergy/tolerance Great capacity to lymphocyte stimulation
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 14 | 21
iDCs (Jakob et al. 1998, Hemmi et al. 2000, Akira
et al. 2001, Iwasaki and Medzhitov 2004). A
modied version of CpGs called CpG-ODN has
been developed for clinical use as an adjuvant
(Cella et al. 1999, Bauer et al. 2001, Coman et
al. 2010).
The CD40 is a costimulatory molecule
expressed on DCs and the interaction with its
ligand CD40L (CD154) leads to an activation of
the NF-κB signaling pathway, a key transducer
of inflammatory signals in DCs (Schulz et al.
2000, Aggarwal 2003, O’Sullivan and Thomas
2003, Ma and Clark 2009). Transfected cells
expressing the CD40L protein can be used in co-
culture assays to induce the maturation of iDCs
(Buelens et al. 2002, Mohty et al. 2003), whereas,
to promote DC activation via CD40 receptor,
some commercial alternatives are available. For
example, CD40L recombinant protein, which
should be used in conjunction with an enhancer for
better performance, is already available (Enzo Life
Sciences, Catalog number: ALX-850-064).
Interleukins and several other molecules can
concomitantly be used to induce DC maturation.
A “maturation cocktail” can, for example, contain
IL-6, IL-1β, TNF-α, IFN-γ, and Prostaglandin E2
(PGE2), (Jonuleit et al. 1997). In the cited case, this
cocktail enhances the pro-inammatory eects of
TNF-α by creating an inammatory environment
that induces DC maturation. This cocktail generates
mature DCs that strongly stimulate the proliferation
of allogeneic lymphocytes (Castiello et al. 2011).
TYPICAL MDDC IMMUNOPHENOTYPE
MARKERS
The phenotype characterization of the obtained
DCs is crucial to demonstrate the success of the
MDDC dierentiation. However, due to the absence
of a consensus concerning which molecules
characterize a DC and which markers are expressed
in dierent DC subsets, it is quite common to nd
a wide variety of markers used in distinct studies.
Nevertheless, the expression of certain molecules
is consistently used to indicate successful MDDC
differentiation and/or maturation. For example,
since a peripheral blood monocyte, the main source
for in vitro DC generation, expresses high levels
of CD14 on its membrane, and MDDC lacks the
expression of this same molecule, CD14 is a marker
for DC dierentiation. Additionally, to determine
the success of the iDC maturation process, it is
essential to monitor the increased expression of
co-stimulatory molecules such as CD40, CD80,
and CD86. These molecules are constitutively
expressed at low levels in DCs, however, their
expression can be considerably increased after
induction of maturation by LPS (Banchereau et al.
2000, Ueno et al. 2007).
The HLA-II molecule is also constitutively
expressed at low levels both in monocytes and DCs.
After a maturation stimulus, its expression on DCs
is increased. Thus, like co-stimulatory molecules,
HLA-II can be used as an indicator of the success
of the iDC maturation process (Al-daccak et al.
2004, Neefjes et al. 2011).
CD1 represents a group of antigen-presenting
molecules which are responsible for the
presentation of lipid antigens and their derivatives.
There are ve isoforms of CD1 proteins [CD1a,
CD1b, CD1c, CD1e (group 1) and CD1d (group
2)]. Myeloid DCs express all ve proteins whereas
Langerhans cells express only CD1a and CD1e
(Mori et al. 2016). In blood monocytes, CD1a,
CD1b, and CD1c (BDCA-1) can be upregulated
on the cell surface by cytokine cocktails designed
to drive DC dierentiation in vitro (Moody 2006).
A strategy for characterizing a pDC, for
example, would encompasses the measurement
of the CD123 (IL-3Rα), CD303 (BDCA-2), and
CD304 (BDCA-4) expression levels. This strategy
depends on the fact that pDCs present high
expression of such molecules in the membrane
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 15 | 21
(Dzionek et al. 2000, Colonna et al. 2004, Swiecki
and Colonna 2015).
Other potential markers are CD207 (Langerin),
which is used to characterize Langerhans cells
(Merad et al. 2008, Nestle et al. 2009), and
CD209 (DC-SIGN), which is expressed on pDCs
and MDDCs and is highly expressed on DCs in
mucosal tissues. The expression of this last marker
is increased after LPS-mediated DC maturation
induction (Belz and Nutt 2012).
Finally, in the analyses performed by flow
cytometry it is of great importance to evaluate the
cellular viability. Substances used to determine
cell viability can be divided into two broad groups:
substances used in live cells or xed cells. Calcein
AM (BD Biosciences, Catalog Number 564061) is a
dye used for staining live cells. The hydrophobicity
of the acetomethoxy (AM) derivative of Calcein
allows this dye to enter on viable cells. Once inside,
intracellular esterases cleave the AM groups o
allowing Calcein to uoresce within the cell. Only
viable cells are stained, since dead cells lack esterase
activity. Calcein AM is optimally excited at the 495
nm wavelength of light and emits maximally at 515
nm (Chung et al. 2017).
7-Amino-actinomycin D, or 7-AAD, is a
nucleic acid dye used to indicate cell viability
in flow cytometric assays. The fluorescence is
detected in the far red range of the spectrum with a
650 nm long-pass lter (Schmid et al. 1992).
For fixed cells, an alternative may be the
reagents BD Horizon FVS from BD Biosciences
(Catalog Number 564406). BD Horizon FVS are
amine-reactive dyes used to discriminate viable
from non-viablecells based on fluorescence
intensity. These dyes react by covalently binding
the cell surface and intracellular amines, resulting
in live cells poorly stained and cells with permeable
membranes exhibiting highly fluorescent.
Typically, dead cells exhibit a uorescence intensity
10 to 20-fold greater than live cells stained with
the same amount of dye. The advantage of these
dyes is that they can be used in cells xed with
formaldehyde, and can be used in protocols where
cell permeabilization occurs (Mahalingaiah et al.
2018).
CONCLUSIONS
Despite several technical challenges, signicant
progress has been made in recent years concerning
the generation of human DCs in vitro. Much of the
recently acquired knowledge was due to studies
using blood monocytes as in vitro precursors of
DCs. The incorporation of knowledge concerning
cellular biology, cytokines, growth factors,
transcription profiles of these cells in different
stages of maturation with the characterization
of the patterns of expression of surface markers
has allowed a more comprehensive and accurate
description of the DCs present in distinct tissues
both in pathological, as well as in healthy situations.
The appropriate combination of cytokines and
growth factors that will generate MDDCs with the
most suitable characteristics for a given study is
already possible. The development of protocols that
allow us to dierentiate MDDCs into a particular
DC subtype as well as to simulate pathological
microenvironments is a reality that has not only
broadened our knowledge but also allows us to
manipulate these cells for use in clinical protocols.
The increasing amounts of reagents available to
induce maturation of iDCs via a specic receptor
also contributes to the production of DCs with
specific characteristics, membrane markers,
specic prole of cytokine secretion, or a given
lymphocyte polarization state.
Another important advance in the study
of MDDCs was the use of multiparametric ow
cytometry (Hasan et al. 2015). The careful selection
of antibodies and the optimal combination of the
uorochromes used to label such specic reagents
are key steps in the establishment of a robust
panel to analyze MDDCs (Maecker et al. 2004,
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 16 | 21
Byrd et al. 2015). To succeed in this step, several
companies provide specic programs to help set
up the analysis panels and protocols to identify the
dierent DC subtypes. International consortia have
also been working for the increased standardization
of flow cytometry protocols including the
EuroFlowConsortium, the Human Immunology
Project Consortium (HIPC), the European
Network for Translational Immunology Research
and Education (ENTIRE) and the Association for
Cancer Immunotherapy (CIMT).
Great progress has been made in the
understanding of the development, dierentiation,
and function of DCs. However, the major challenge
will be to transpose the acquired knowledge into in
vivo situations to elucidate the DCs physiological
behavior. This knowledge will be useful in the design
of novel vaccines and DC-based immunotherapies
for the prevention and treatment of several human
diseases.
ACKNOWLEDGMENTS
This work was supported by the Coordenação de
Aperfeiçoamento de Pessoal de Nível Superior
(CAPES).
AUTHOR CONTRIBUTIONS
Both authors wrote and revised the manuscript.
REFERENCES
AGGARWAL BB. 2003. Signalling pathways of the TNF
superfamily: a double-edged sword. Nat Rev Immunol 3:
745-756.
AKIRA S AND TAKEDA K. 2004. Toll-like receptor
signalling. Nat Rev Immunol 4: 499-511.
AKIRA S, TAKEDA K AND KAISHO T. 2001. Toll-like
receptors: critical proteins linking innate and acquired
immunity. Nat Immunol 2: 675-680.
AL-DACCAK R, MOONEY N AND CHARRON D. 2004.
MHC class II signaling in antigen-presenting cells. Curr
Opin Immunol 16: 108-113.
ANTON D, DABADGHAO S, PALUCKA K, HOLM G AND
YI Q. 1998. Generation of dendritic cells from peripheral
blood adherent cells in medium with human serum. Scand
J Immunol 47: 116-121.
ARDAVIN C, WU L, LI CL AND SHORTMAN K. 1993.
Thymic dendritic cells and T cells develop simultaneously
in the thymus from a common precursor population.
Nature 362: 761-763.
BANCHEREAU J, BRIERE F, CAUX C, DAVOUST
J, LEBECQUE S, LIU YJ, PULENDRAN B AND
PALUCKA K. 2000. Immunobiology of dendritic cells.
Annu Rev Immunol 18: 767-811.
BANCHEREAU J ET AL. 2001. Immune and clinical
responses in patients with metastatic melanoma to
CD34(+) progenitor-derived dendritic cell vaccine. Cancer
Res 61: 6451-6458.
BARKER K. 1998. At the Bench: A Laboratory Navigator.
Cold Spring Harbor Laboratory Press, 465p.
BAUER M, REDECKE V, ELLWART JW, SCHERER B,
KREMER JP, WAGNER H AND LIPFORD GB. 2001.
Bacterial CpG-DNA triggers activation and maturation of
human CD11c-, CD123+ dendritic cells. J Immunol 166:
5000-5007.
BAUER T, ZAGÓRSKA A, JURKIN J, YASMIN N, KÖFFEL
R, RICHTER S, GESSLBAUER B, LEMKE G AND
STROBL H. 2012. Identification of Axl as a downstream
effector of TGF-β1 during Langerhans cell differentiation
and epidermal homeostasis. J Exp Med 209: 2033-2047.
BELZ GT AND NUTT SL. 2012. Transcriptional programming
of the dendritic cell NETWORK. Nat Rev Immunol 12:
101-113.
BIRON CA. 2001. Interferons alpha and beta as immune
regulators-a new look. Immunity 14: 661-664.
BRENNER D, BLASER H AND MAK TW. 2015. Regulation
of tumour necrosis factor signalling: live or let die. Nat
Rev Immunol 15: 362-374.
BUELENS C, BARTHOLOMÉ EJ, AMRAOUI Z,
BOUTRIAUX M, SALMON I, THIELEMANS K,
WILLEMS F AND GOLDMAN M. 2002. Interleukin-3
and interferon beta cooperate to induce differentiation of
monocytes into dendritic cells with potent helper T-cell
stimulatory properties. Blood 99: 993-998.
BYRD T, CARR KD, NORMAN JC, HUYE L AND HEGDE
M. 2015. Polystyrene microspheres enable 10-color
compensation for immunophenotyping of primary human
leukocytes. Cytometry A 87: 1038-1046.
CASTIELLO L, SABATINO M, JIN P, CLAYBERGER C,
MARINCOLA FM, KRENSKY AM AND STRONCEK
DF. 2011. Monocyte-derived DC maturation strategies and
related pathways: a transcriptional view. Cancer Immunol
Immunother 60: 457-466.
CELLA M, SALIO M, SAKAKIBARA Y, LANGEN
H, JULKUNEN I AND LANZAVECCHIA A. 1999.
Maturation, activation, and protection of dendritic cells
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 17 | 21
induced by double-stranded RNA. J Exp Med 189: 821-
829.
CHALLAGUNDLA KB ET AL. 2015. Exosome-mediated
transfer of microRNAs within the tumor microenvironment
and neuroblastoma resistance to chemotherapy. J Natl
Cancer Inst 7: djv135.
CHOMARAT P, DANTIN C, BENNETT L, BANCHEREAU
J AND PALUCKA AK. 2003. TNF skews monocyte
differentiation from macrophages to dendritic cells. J
Immunol 171: 2262-2269.
CHUNG S, NGUYEN V, LIN YL, KAMEN L AND SONG A.
2017. Thaw-and-use target cells pre-labeled with calcein
AM for antibody-dependent cell-mediated cytotoxicity
assays. J Immunol Methods 447: 37-46.
CIMT - ASSOCIATION FOR CANCER IMMUNOTHERAPY.
http://www.cimt.eu/workgroups/cip/proficiency
(Accessed 12 June 2018).
CLICK RE. 2014. Review: 2-mercaptoethanol alteration of
in vitro immune functions of species other than murine. J
Immunol Methods 402: 1-8.
COFFMAN RL, SHER A AND SEDER RA. 2010. Vaccine
adjuvants: putting innate immunity to work. Immunity 33:
492-503.
COLLIN M AND BIGLEY V. 2018. Human dendritic cell
subsets: an update. Immunology 154: 3-20.
COLONNA M, TRINCHIERI G AND LIU YJ. 2004.
Plasmacytoid dendritic cells in immunity. Nat Immuno l5:
1219-1226.
DALOD M, CHELBI R, MALISSEN B AND LAWRENCE T.
2014. Dendritic cell maturation: functional specialization
through signaling specificity and transcriptional
programming. EMBO J 33: 1104-1116.
DAUER M, OBERMAIER B, HERTEN J, HAERLE C,
POHL K, ROTHENFUSSER S, SCHNURR M, ENDRES
S AND EIGLER A. 2003. Mature dendritic cells derived
from human monocytes within 48 hours: a novel strategy
for dendritic cell differentiation from blood precursors. J
Immunol 170: 4069-4076.
DELIREZH N, SHOJAEEFAR E, PARVINP AND ASADI B.
2013. Comparison the effects of two monocyte isolation
methods, plastic adherence and magnetic activated cell
sorting methods, on phagocytic activity of generated
dendritic cells. Cell J 15: 218-223.
DIETZ AB, BULUR PA, EMERY RL, WINTERS JL, EPPS
DE, ZUBAIR AC AND VUK-PAVLOVIĆ S. 2006. A
novel source of viable peripheral blood mononuclear cells
from leukoreduction system chambers. Transfusion 46:
2083-2089.
DUBOIS S, MARINER J, WALDMANN TA AND TAGAYA
Y. 2002. IL-15Ralpha recycles and presents IL-15 In trans
to neighboring cells. Immunity 17: 537-547.
DUBOIS SP, WALDMANN TA AND MÜLLER JR. 2005.
Survival adjustment of mature dendritic cells by IL-15.
Proc Natl Acad Sci USA 102: 8662-8667.
DUPERRIER K, ELJAAFARI A, DEZUTTER-
DAMBUYANT C, BARDIN C, JACQUET C, YONEDA
K, SCHMITT D, GEBUHRER L AND RIGALD. 2000.
Distinct subsets of dendritic cells resembling dermal DCs
can be generated in vitro from monocytes, in the presence
of different serum supplements. J Immunol Methods 238:
119-131.
DZIONEK A, FUCHS A, SCHMIDT P, CREMER S, ZYSK
M, MILTENYI S, BUCK DW AND SCHMITZ J. 2000.
BDCA-2, BDCA-3, and BDCA-4: three markers for
distinct subsets of dendritic cells in human peripheral
blood. J Immunol 165: 6037-6046.
EBNER S, HOFER S, NGUYEN VA, FÜRHAPTER C,
HEROLD M, FRITSCH P, HEUFLER C AND ROMANI
N. 2002. A novel role for IL-3: human monocytes cultured
in the presence of IL-3 and IL-4 differentiate into dendritic
cells that produce less IL-12 and shift Th cell responses
toward a Th2 cytokine pattern. J Immunol 168: 6199-6207.
EBNER S, NEYER S, HOFER S, NUSSBAUMER W,
ROMANI N AND HEUFLER C. 2001. Generation of
large numbers of human dendritic cells from whole blood
passaged through leukocyte removal filters: an alternative
to standard buffy coats. J Immunol Methods 252: 93-104.
ELKORD E, WILLIAMS PE, KYNASTON H AND
ROWBOTTOM AW. 2005. Human monocyte isolation
methods influence cytokine production from in vitro
generated dendritic cells. Immunology 114: 204-212.
ESEBANMEN GE AND LANGRIDGE WHR. 2017. The role
of TGF-beta signaling in dendritic cell tolerance. Immunol
Res 65: 987-994.
EuroFlow. 2018. https://euroflow.org/usr/pub/prlogin.php.
(accessed 03 May 2018).
ENTIRE - EUROPEAN NETWORK FOR
TRANSLATIONAL IMMUNOLOGY RESEARCH
AND EDUCATION. 2018. http://www.cost.eu/COST_
Actions/bmbs/BM0907?parties (accessed 03 May 2018).
GALLUCCI S, LOLKEMA M AND MATZINGER P. 1999.
Natural adjuvants: endogenous activators of dendritic
cells. Nat Med 5: 1249-1255.
GANDHI RT ET AL. 2016. Immunization of HIV-1-Infected
Persons with Autologous Dendritic Cells Transfected
With mRNA Encoding HIV-1 Gag and Nef: Results of a
Randomized, Placebo-Controlled Clinical Trial. J Acquir
Immune Defic Syndr 71: 246-253.
GARG AD, COULIE PG, VAN DEN EYNDE BJ AND
AGOSTINIS P. 2017. Integrating Next-Generation
Dendritic Cell Vaccines into the Current Cancer
Immunotherapy Landscape. Trends Immunol 38: 577-593.
GEISSMANN F, PROST C, MONNET JP, DY M, BROUSSE
N AND HERMINE O. 1998. Transforming growth factor
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 18 | 21
beta 1, in the presence of granulocyte/macrophage colony-
stimulating factor and interleukin 4, induces differentiation
of human peripheral blood monocytes into dendritic
Langerhans cells. J Exp Med 187: 961-966.
GILLIO-MEINA C, CEPINSKAS G, CECCHINI EL AND
FRASER DD. 2013. Translational research in pediatrics
II: blood collection, processing, shipping, and storage.
Pediatrics 131: 754-766.
GRANOT ET AL. 2017. Dendritic Cells Display Subset and
Tissue-Specific Maturation Dynamics over Human Life.
Immunity 46: 504-515.
GUERMONPREZ P, VALLADEAU J, ZITVOGEL L, THÉRY
C AND AMIGORENA S. 2002. Antigen presentation and
T cell stimulation by dendritic cells. Annu Rev Immunol
20: 621-667.
HAENSSLE H, BUHL T, KNUDSEN S, KRUEGER U,
ROSENBERGER A, REICH K AND NEUMANN C.
2008. CD40 ligation during dendritic cell maturation
reduces cell death and prevents interleukin-10-induced
regression to macrophage-like monocytes. Exp Dermatol
17: 177-187.
HAMMER GE AND MA A. 2013. Molecular control of steady-
state dendritic cell maturation and immune homeostasis.
Annu Rev Immunol 31: 743-791.
HASAN M ET AL. 2015. Semi-automated and standardized
cytometric procedures for multi-panel and multi-
parametric whole blood immunophenotyping. Clin
Immunol 157: 261-276.
HEIL F, HEMMI H, HOCHREIN H, AMPENBERGER F,
KIRSCHNING C, AKIRA S, LIPFORD G, WAGNER H
AND BAUER S. 2004. Species-specific recognition of
single-stranded RNA via toll-like receptor 7 and 8. Science
303: 1526-1529.
HEMMI H ET AL. 2000. A Toll-like receptor recognizes
bacterial DNA. Nature 408: 740-745.
HOLMES KL, FONTES B, HOGARTH P, KONZ R,
MONARD S, PLETCHER CHJR, WADLEY RB,
SCHMID I AND PERFETTO SP. 2014. International
Society for the Advancement of Cytometry cell sorter
biosafety standards. Cytometry A 85: 434-453.
ITO T, AMAKAWA R, INABA M, IKEHARA S, INABA K
AND FUKUHARA S. 2001. Differential regulation of
human blood dendritic cell subsets by IFNs. J Immunol
166: 2961-2969.
ITO T, AMAKAWA R, KAISHO T, HEMMI H, TAJIMA K,
UEHIRA K, OZAKI Y, TOMIZAWA H, AKIRA S AND
FUKUHARA S. 2002. Interferon-alpha and interleukin-12
are induced differentially by Toll-like receptor 7 ligands
in human blood dendritic cell subsets. J Exp Med 195:
1507-1512.
ITO Y AND SHINOMIYA K. 2001. A new continuous-flow
cell separation method based on cell density: principle,
apparatus, and preliminary application to separation of
human buffy coat. J Clin Apher 16: 186-191.
IWAMOTO S, IWAI S, TSUJIYAMA K, KURAHASHI C,
TAKESHITA K, NAOE M, MASUNAGA A, OGAWA
Y, OGUCHI K AND MIYAZAKI A. 2007. TNF-alpha
drives human CD14+ monocytes to differentiate into
CD70+ dendritic cells evoking Th1 and Th17 responses. J
Immunol 179: 1449-1457.
IWASAKI A AND MEDZHITOV R. 2004. Toll-like receptor
control of the adaptive immune responses. Nat Immunol
5: 987-995.
JAKOB T, WALKER PS, KRIEG AM, UDEY MC AND
VOGEL JC. 1998. Activation of cutaneous dendritic cells
by CpG-containing oligodeoxynucleotides: a role for
dendritic cells in the augmentation of Th1 responses by
immunostimulatory DNA. J Immunol 161: 3042-3049.
JANETZKI S ET AL. 2010. Performance of serum-
supplemented and serum-free media in IFN gamma Elispot
Assays for human T cells. Cancer Immunol Immunother
59: 609-618.
JANSEN JH, WIENTJENS GJ, FIBBE WE, WILLEMZE
R AND KLUIN-NELEMANS HC. 1989. Inhibition of
human macrophage colony formation by interleukin 4. J
Exp Med 170: 577-582.
JOFFRE O, NOLTE MA, SPÖRRI R AND REIS E SOUSA C.
2009. Inflammatory signals in dendritic cell activation and
the induction of adaptive immunity. Immunol Rev 227:
234-247.
JOFFRE OP, SEGURA E, SAVINA A AND AMIGORENA
S. 2012. Cross-presentation by dendritic cells. Nat Rev
Immunol 12: 557-569.
JONULEIT H, KÜHN U, MÜLLER G, STEINBRINK K,
PARAGNIK L, SCHMITT E, KNOP J AND ENK AH.
1997. Pro-inflammatory cytokines and prostaglandins
induce maturation of potent immunostimulatory dendritic
cells under fetal calf serum-free conditions. Eur J Immunol
27: 3135-3142.
KAVANAGH DG AND BHARDWAJ N. 2002. A division
of labor: DC subsets and HIV receptor diversity. Nat
Immunol 3: 891-893.
KURTS C, ROBINSON BW AND KNOLLE PA. 2010. Cross-
priming in health and disease. Nat Rev Immunol 10: 403-
414.
LEHNER M AND HOLTER W.2002. Endotoxin-free
purification of monocytes for dendritic cell generation
via discontinuous density gradient centrifugation based
on diluted ficoll-paque plus. Int. Arch Allergy Immunol
128: 73-76.
LI DY, GU C, MIN J, CHU ZH AND OU QJ. 2012. Maturation
induction of human peripheral blood mononuclear cell-
derived dendritic cells. Exp Ther Med 4: 131-134.
LI MO, WAN YY, SANJABI S, ROBERTSON AK AND
FLAVELL RAL. 2006. Transforming growth factor-beta
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 19 | 21
regulation of immune responses. Annu RevImmunol 24:
99-146.
LIEW FY, XU D, BRINT EK AND O’NEILL LA. 2005.
Negative regulation of toll-like receptor-mediated immune
responses. Nat Rev Immunol 5: 446-458.
LIU YJ. 2005. IPC: professional type 1 interferon-producing
cells and plasmacytoid dendritic cell precursors. Annu Rev
Immunol 23: 275-306.
LUTZ MB AND SCHULER G. 2002. Immature, semi-mature
and fully mature dendritic cells: which signals induce
tolerance or immunity? Trends Immunol 23: 445-449.
MA DY AND CLARK EA. 2009. The role of CD40 and
CD154/CD40L in dendritic cells. Semin Immunol 21:
265-272.
MAECKER HT, FREY T, NOMURA LE AND TROTTER J.
2004. Selecting fluorochrome conjugates for maximum
sensitivity. Cytometry A 62: 169-173.
MAHALINGAIAH PK, PALENSKI T AND VAN VLEET
TR. 2018. An In Vitro Model of Hematotoxicity:
Differentiation of Bone Marrow-Derived Stem/Progenitor
Cells into Hematopoietic Lineages and Evaluation of
Lineage-Specific Hematotoxicity. Curr Protoc Toxicol 1:
e45.
MASSA C AND SELIGER B. 2013. Fast dendritic cells
stimulated with alternative maturation mixtures induce
polyfunctional and long-lasting activation of innate and
adaptive effector cells with tumor-killing capabilities. J
Immuno l190: 3328-3337.
MATZINGER P. 1994. Tolerance, danger, and the extended
family. Annu Rev Immunol 12: 991-1045.
MERAD M, GINHOUX F AND COLLIN M. 2008. Origin,
homeostasis and function of Langerhans cells and other
langerin-expressing dendritic cells. Nat Rev Immunol 8:
935-947.
MERAD M, SATHE P, HELFT J, MILLER J AND MORTHA
A. 2013. The dendritic cell lineage: ontogeny and function
of dendritic cells and their subsets in the steady state and
the inflamed setting. Annu Rev Immunol 31: 563-604.
MEYER TP, ZEHNTER I, HOFMANN B, ZAISSERER J,
BURKHART J, RAPP S, WEINAUER F, SCHMITZ
J AND ILLERT WE. 2005. Buffy Coats (FBC): a
source of peripheral blood leukocytes recovered from
leukocytedepletion filters. J Immunol Methods 20: 150-
166.
MIAO EA AND WARREN SE. 2010. Innate immune
detection of bacterial virulence factors via the NLRC4
inflammasome. J Clin Immunol 30: 502-506.
MOHAMADZADEH M, BERARD F, ESSERT G,
CHALOUNI C, PULENDRAN B, DAVOUST J,
BRIDGES G, PALUCKA AK AND BANCHEREAU J.
2001. Interleukin 15 skews monocyte differentiation into
dendritic cells with features of Langerhans cells. J Exp
Med 194: 1013-1020.
MOHTY M, VIALLE-CASTELLANO A, NUNES JA,
ISNARDON D, OLIVE D AND GAUGLER B. 2003.
IFN-alpha skews monocyte differentiation into Toll-like
receptor 7-expressing dendritic cells with potent functional
activities. J Immunol 171: 3385-3393.
MOODY DB. 2006. TLR gateways to CD1 function. Nat
Immunol 7: 811-817.
MOORE GE, GERNER RE AND FRANKLIN HA. 1967.
Culture of normal human leukocytes. JAMA 199: 519-
524.
MORI L, LEPORE M AND DE LIBERO G. 2016. The
Immunology of CD1- and MR1-Restricted T Cells. Annu
Rev Immunol 34: 479-510.
NAPOLITANI G, LEPORE M AND DE LIBERO G. 2005.
The Immunology of CD1- and MR1-Restricted T Cells.
Nat Immunol 6: 769-776.
NEEFJES J, JONGSMA ML, PAUL P AND BAKKE O. 2011.
Towards a system understanding of MHC class I and MHC
class II antigen presentation. Nat Rev Immunol 11: 823-
836.
NESTLE FO, DI MEGLIO P, QIN JZ AND NICKOLOFF BJ.
2009. Skin immune sentinels in health and disease. Nat
Rev Immunol 9: 679-691.
OBERMAIER B, DAUER M, HERTEN J, SCHAD K,
ENDRES S AND EIGLER A. 2003. Development of a
new protocol for 2-day generation of mature dendritic cells
from human monocytes. Biol Proced Online 5: 197-203.
O’SULLIVAN B AND THOMAS R. 2003. CD40 and dendritic
cell function. Crit Rev Immunol 23: 83-107.
PARK BS AND LEE JO. 2013. Recognition of
lipopolysaccharide pattern by TLR4 complexes. Exp Mol
Med 6: 45-66.
PATARROYO M, PRIETO J, BEATTY PG, CLARK EA AND
GAHMBERG CG. 1988. Adhesion-mediating molecules
of human monocytes. Cell Immunol 113: 278-289.
PATEL AA ET AL. 2017. The fate and lifespan of human
monocyte subsets in steady state and systemic
inflammation. J Exp Med 214: 1913-1923.
PAVLOVIĆ B, TOMIĆ S, ĐOKIĆ J, VASILIJIĆ S, VUČEVIĆ
D, LUKIĆ J, GRUDEN-MOVSESIJAN A, ILIĆ N,
MARKOVIĆ M AND ČOLIĆ M. 2015. Fast dendritic
cells matured with Poly (I:C) may acquire tolerogenic
properties. Cytotherapy 17: 1763-1776.
PENG JC, THOMAS R ANDNIELSEN LK. 2005. Generation
and maturation of dendritic cells for clinical application
under serum-free conditions. J Immunother 28: 599-609.
PERUSSIA B, FANNING V AND TRINCHIERI G. 1985. A
leukocyte subset bearing HLA-DR antigens is responsible
for in vitro alpha interferon production in response to
viruses. Nat Immun Cell Growth Regul 4: 120-137.
PULENDRAN B. 2015. The Varieties of Immunological
Experience: Of Pathogens, Stress, and Dendritic Cells.
Annu Rev Immunol 33: 563-606.
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 20 | 21
RAETZ CR AND WHITFIELD C. 2002. Lipopolysaccharide
endotoxins. Annu Rev Biochem 71: 635-700.
RAMADAN G, KONINGS S, KURUP VP AND KEEVER-
TAYLOR CA. 2004. Generation of Aspergillus- and
CMV- specific T-cell responses using autologous fast DC.
Cytotherapy 6: 223-234.
RANDOLPH GJ, OCHANDO J AND PARTIDA-SÁNCHEZ
S.2008. Migration of dendritic cell subsets and their
precursors. Annu Rev Immunol 26: 293-316.
RELLOSO M, PUIG-KRÖGER A, PELLO OM,
RODRÍGUEZ-FERNÁNDEZ JL, DE LA ROSA G,
LONGO N, NAVARRO J, MUÑOZ-FERNÁNDEZ
MA, SÁNCHEZ-MATEOS P AND CORBÍ AL. 2002.
DC-SIGN (CD209) expression is IL-4 dependent and
is negatively regulated by IFN, TGF-beta, and anti-
inflammatory agents. J Immunol 168: 2634-2643.
REPNIK U, KNEZEVIC M AND JERAS M. 2003. Simple
and cost-effective isolation of monocytes from buffy coats.
J Immunol Methods 278: 283-292.
ROJAS-CANALES D, KRISHNAN R, JESSUP CF AND
COATES PT. 2012. Early exposure of interferon-γ inhibits
signal transducer and activator of transcription-6 signalling
and nuclear factor κB activation in a short-term monocyte-
derived dendritic cell culture promoting ‘FAST’ regulatory
dendritic cells. Clin Exp Immunol 167: 447-458.
RUPPERT J, FRIEDRICHS D, XU H AND PETERS JH.
1991. IL-4 decreases the expression of the monocyte
differentiation marker CD14, paralleled by an increasing
accessory potency. Immunobiology 182: 449-464.
SAIKH KU, KHAN AS, KISSNER T AND ULRICH RG.
2001. IL-15-induced conversion of monocytes to mature
dendritic cells. Clin Exp Immunol 126: 447-455.
SALLUSTO F AND LANZAVECCHIA A. 1994. Efficient
presentation of soluble antigen by cultured human dendritic
cells is maintained by granulocyte/macrophage colony-
stimulating factor plus interleukin 4 and downregulated by
tumor necrosis factor alpha. J Exp Med 179: 1109-1118.
SANARICO N, CIARAMELLA A, SACCHI A,
BERNASCONI D, BOSSÙ P, MARIANI F, COLIZZI
V AND VENDETTI S. 2006. Human monocyte-derived
dendritic cells differentiated in the presence of IL-2
produce proinflammatory cytokines and prime Th1
immune response. J Leukoc Biol 80: 555-562.
SANDER J ET AL. 2017. Cellular Differentiation of Human
Monocytes Is Regulated by Time-Dependent Interleukin-4
Signaling and the Transcriptional Regulator NCOR2.
Immunity 47: 1051-1066.
SANTINI SM, LAPENTA C, LOGOZZI M, PARLATO S,
SPADA M, DI PUCCHIO T AND BELARDELLI F. 2000.
Type I interferon as a powerful adjuvant for monocyte-
derived dendritic cell development and activity in vitro and
in Hu-PBL-SCID mice. J Exp Med 191: 1777-1788.
SCHLEE M AND HARTMANN G. 2016. Discriminating self
from non-self in nucleic acid sensing. Nat Rev Immunol
16: 566-580.
SCHMID I, KRALL WJ, UITTENBOGAART CH, BRAUN
J AND GIORGI JV. 1992. Dead cell discrimination with
7-amino-actinomycin D in combination with dual color
immunofluorescence in single laser flow cytometry.
Cytometry 13: 204-208.
SCHULZ O, EDWARDS AD, SCHITO M, ALIBERTI J,
MANICKASINGHAM S, SHER A AND REIS E SOUSA
C. 2000. CD40 triggering of heterodimeric IL-12 p70
production by dendritic cells in vivo requires a microbial
priming signal. Immunity 13: 453-462.
SEGURA E, VALLADEAU-GUILEMOND J, DONNADIEU
MH, SASTRE-GARAU X, SOUMELIS V AND
AMIGORENA S. 2012. Characterization of resident and
migratory dendritic cells in human lymph nodes. J Exp
Med 209: 653-660.
STEINMAN RM. 2012. Decisions about dendritic cells: past,
present, and future. Annu Rev Immunol 30: 1-22.
STEINMAN RM AND COHN ZA. 1973. Identification of a
novel cell type in peripheral lymphoid organs of mice. I.
Morphology, quantitation, tissue distribution. J Exp Med
137: 1142-1462.
STEINMAN RM AND WITMER MD. 1978. Lymphoid
dendritic cells are potent stimulators of the primary mixed
leukocyte reaction in mice. Proc Natl Acad Sci USA 75:
5132-5136.
STOCKWIN LH, MCGONAGLE D, MARTIN IG AND
BLAIR GE. 2000. Dendritic cells: immunological
sentinels with a central role in health and disease. Immunol
Cell Biol 78: 91-102.
STRASSER EF AND ECKSTEIN R. 2010. Optimization of
leukocyte collection and monocyte isolation for dendritic
cell culture. Transfus Med Rev 24: 130-139.
STRASSER EF, WEIDINGER T, ZIMMERMANN R,
RINGWALD J AND ECKSTEIN R. 2007. Recovery
of white blood cells and platelets from leukoreduction
system chambers of Trima Accel and COBE Spectra
plateletpheresis devices. Transfusion 47: 1943-1944
SUZUKI H ET AL. 2004. Activities of granulocyte-
macrophage colony-stimulating factor and interleukin-3
on monocytes. Am J Hematol 75: 179-189.
SWIECKI M AND COLONNA M. 2015. The multifaceted
biology of plasmacytoid dendritic cells. Nat Rev Immunol
15: 471-485.
TAKAHASHI K, HONEYMAN MC AND HARRISON
LC. 1997. Dendritic cells generated from human blood
in granulocyte macrophage-colony stimulating factor and
interleukin-7. Hum Immunol 55: 103-116.
TANAKA F, YAMAGUCHI H, HARAGUCHI N, MASHINO
K, OHTA M, INOUE H AND MORI M. 2006. Efficient
induction of specific cytotoxic T lymphocytes to tumor
rejection peptide using functional matured 2 day-cultured
GIOVANA CECHIM and JOSÉ A.B. CHIES HUMAN MONOCYTE-DERIVED DENTRITIC CELLS GENERATION
An Acad Bras Cienc (2019) 91(4) e20190310 21 | 21
dendritic cells derived from human monocytes. Int J Oncol
29: 1263-1268.
TRAVIS MA AND SHEPPARD D. 2014. TGF-β activation
and function in immunity. Annu Rev Immunol 32: 51-82.
TURVILLE SG, CAMERON PU, HANDLEY A, LIN G,
PÖHLMANN S, DOMS RW AND CUNNINGHAM AL.
2002. Diversity of receptors binding HIV on dendritic cell
subsets. Nat Immunol 3: 975-983.
UENO H ET AL. 2007. Dendritic cell subsets in health and
disease. Immunol Rev 219: 118-142.
ULANOVA M, TARKOWSKI A, HAHN-ZORIC M AND
HANSON LA. 2001. The Common vaccine adjuvant
aluminum hydroxide up-regulates accessory properties
of human monocytes via an interleukin-4-dependent
mechanism. Infect Immun 69: 1151-1159.
VATSAN RS, BROSS PF, LIU K, THEORET M, DE CLARO
AR, LU J, HELMS W, NILAND B, HUSAIN SR AND
PURI RK. 2013. Regulation of immunotherapeutic
products for cancer and FDA’s role in product development
and clinical evaluation. J Immunother Cancer 29: 1-5.
VICENTE-SUAREZ I, BRAYER J, VILLAGRA A, CHENG
F AND SOTOMAYOR EM. 2009. TLR5 ligation by
flagellin converts tolerogenic dendritic cells into activating
antigen-presenting cells that preferentially induce T-helper
1 responses. Immunol Lett 125: 114-118.
VOGT TK, LINK A, PERRIN J, FINKE D AND LUTHER
SA. 2009. Novel function for interleukin-7 in dendritic cell
development. Blood 113: 3961-3968.
WAJANT H, PFIZENMAIER K AND SCHEURICH P. 2003.
Tumor necrosis factor signaling. Cell Death Differ 10: 45-65.
WANG S AND EL-DEIRY WS. 2003. TRAIL and apoptosis
induction by TNF-family death receptors. Oncogene 22:
8628-8633.
WHO - WORLD HEALTH ORGANIZATION. 2010.
Guidelines on Drawing Blood: Best Practices in
Phlebotomy. http://www.euro.who.int/__data/assets/pdf_
file/0005/268790/WHO-guidelines-on-drawing-blood-
best-practices-in-phlebotomy-Eng.pdf?ua-1 (accessed 12
April 2018).
WORBS T. 2017. Dendritic cell migration in health and
disease. Nat Rev Immunol 17: 30-48.
WU L AND LIU YJ. 2007. Development of dendritic-cell
lineages. Immunity 26: 741-750.
XIAO Q, LI X, SUN D, YI H, LU X AND NIAN H. 2016. TLR7
Engagement on Dendritic Cells Enhances Autoreactive
Th17 Responses via Activation of ERK. J Immunol 197:
3820-3830.
YANG GX, LIAN ZX, KIKUCHI K, MORITOKI Y, ANSARI
AA, LIU YJ, IKEHARA S AND GERSHWIN ME. 2005.
Plasmacytoid dendritic cells of different origins have
distinct characteristics and function: studies of lymphoid
progenitors versus myeloid progenitors. J Immunol 175:
7281-7287.
