#
Development of a standardized protocol for reproducible generation of
matured monocyte-derived dendritic cells suitable for clinical application
H.R. Bohnenkamp and T. Noll*
Institute of Biotechnology 2, Research Center Juelich GmbH, 52428 Juelich, Germany; *Author for
Received 21 March 2003; accepted in revised form 3 September 2003
Key words: Dendritic cells, Immunotherapy, Monocyte enrichment, Serum-free
Abstract
There is increasing interest in the generation of dendritic cells (DC) for cancer immunotherapy. In order to
utilize DC in clinical trials it is necessary to have standardized, reproducible and easy to use protocols. We
describe here the process development for the generation of DC as the result of investigation of culture
conditions as well as consumption rates of medium and cytokines. Our studies demonstrate that highly
viable DC (93 ± 2%) can be produced from CD14
+
enriched monocytes via immunomagnetic beads in a high
yield (31 ± 6%) with X-VIVO 15, 400 U ml
1
GM–CSF and 2000 U ml
1
IL-4 without serum and feeding.
For the maturation of DC different cocktails (TNF-, IL-1, IL-6, PGE
2
and TNF-, PGE
2
) were
compared. In both cases cells expressed typical surface molecules of mature DC and induced high prolif-
erative responses in mixed lymphocyte reactions which led to IFN- producing T-lymphocytes. The data
suggest that the use of this optimized, easy to use protocol results in highly mature DC.
Abbreviations: DC – dendritic cells; moDC – monocytes derived dendritic cells; FACS – fluorescence
activated cell sorter; FCS – fetal calf serum; GM–CSF – granulocyte macrophage–colony stimulating
factor; MLR – mixed lymphocyte reaction; PBMC – peripheral blood mononuclear cell; IL – Interleukin;
PGE
2
– Prostaglandin E
2
; TNF – tumor necrosis factor
Introduction
Dendritic cells (DC) are professional antigen
presenting cells, inducing immune responses while
stimulating na
€
ve T-lymphocytes and controlling
the activation of T-helper cells in the T
H
1–T
H
2
pathway (Banchereau and Steinman 1998;
O’Garra and Arai 2000).
The use of these potent stimulators as vaccines
for the immunotherapy of cancer is a very promis-
ing approach to overcome the tumor escape in
immune surveillance (Costello et al. 1999). To
date, several clinical trials have been performed
utilizing DC preparations, which demonstrated
anti-tumor responses (Nestle et al. 1998; Thurner
et al. 1999a; Kugler et al. 2000; Fong et al. 2001;
Kikuchi et al. 2001; Kobayashi et al. 2001; Schuler-
Thurner et al. 2002).
Different sources can be used for the generation
of DC: proliferating CD34
+
precursors in blood
(Caux et al. 1992) after G-CSF mobilization
(Mackensen et al. 2000), non-proliferating CD14
+
monocytes in peripheral blood after enrichment via
magnetic beads (Pickl et al. 1996; Dietz et al. 2000)
or adhesion (Bender et al. 1996; Romani et al. 1996;
Thurner et al. 1999b; Berger et al. 2002) and enrich-
ment of DC after cultivation of PBMC via elutria-
tion (Bernard et al. 1998; Goxe et al. 2000). Rare
circulating DC can also be isolated, but although
patients can be pretreated with Flt3 ligand the yield
121
Cytotechnology 121–131, 2003.
42:
2003 Kluwer Academic Publishers. Printed in the Netherlands.
of DC is comparably small (Fong et al. 2001). While
DC from CD34
+
cells require a prolonged culture
and special cytokine setup in order to increase
the small number of precursors, monocyte-derived
DC (moDC) are easy to obtain after enrichment
of monocytes by magnetic separation or adherence,
followed by differentiation using granulocyte
macrophage–colony stimulating factor (GM–
CSF) and interleukin-4 (IL-4). This method devel-
oped by Sallusto and Lanzavecchia (1994) and
Romani et al. (1994) is applied widely in experi-
mental protocols. Due to the ease of availability
of monocytes, rapidness of fully differentiation to
unmature (5–6 days) or mature (6–8 days) DC using
special maturation cocktails (e.g., TNF-,IL-1,
IL-6, PGE
2
(Jonuleit et al. 1997) or TNF-, PGE
2
(Kalinski et al. 1998)) and the possibility of avoid-
ing foreign antigens (fetal calf serum (FCS)), this
protocol was adapted for clinical applications
(Thurner et al. 1999b). For studies where DCs are
to be cultured ex vivo and then reintroduced to
the patient, it is advisable to avoid FCS or any
kind of foreign antigen due to possible infections
and immunogenicity. However, the use of autolo-
gous serum or plasma may cause non-standardized
cultivation conditions and so is best to avoid.
Here, we describe the development of a standard-
ized protocol, which circumvents the disadvantages
of non-uniform culture conditions caused by sup-
plemented serum, non-defined cell densities and
adherence steps. Furthermore, the protocol elimi-
nates the need for feeding the cells, which increases
the risk of contamination. In this study, we have
examined the influence of several important culti-
vation parameters to optimize and setup the serum-
free generation of DC in X-VIVO 15. Cell density,
GM–CSF and IL-4 concentration, medium com-
ponents like glucose, lactate and amino acids were
assessed. Moreover the influence of different
maturation cocktails (TNF-,IL-1, IL-6, PGE
2
and TNF-, PGE
2
) on the maturation status of DC
was investigated.
Material and methods
Medium, serum and cytokines
As standard medium X-VIVO 15 (Bio Whittaker,
Walkersville, MD) was used for the generation of
DC. RPMI 1640 (Gibco BRL, Eggenstein,
Germany) supplemented with 10% heat-
inactivated (56
C, 30 min) FCS (Gibco BRL,
Eggenstein, Germany) was used for the mixed
lymphocyte reaction (MLR). The differentiation of
monocytes into DC was performed using rhuGM–
CSF (Leucomax
TM
, Norvartis, Nuernberg,
Germany) and rhuIL-4 (R&D, Wiesbaden,
Germany). Factors added for maturation of DC
included rhuTNF-, rhuIL-1, rhuIL-6 (all from
R&D) and PGE
2
(Sigma, Deisenhofen, Germany).
For the MLR rhuIL-2 (from BHK 21 cells, kindly
provided by Dr Wagner, GBF, Braunschweig,
Germany) was utilized.
Generation of human monocyte derived
dendritic cells
Peripheral blood mononuclear cells (PBMC) were
obtained from buffy coat preparations from
healthy donors (kindly provided by Dr T. Tonn,
Blutspendedienst Hessen, Germany) by standard
density gradient centrifugation on Biocoll (Ficoll
separating solution) (Biochrom KG, Berlin,
Germany). Monocytes CD14
+
were affinity-
purified utilizing the MACS
TM
CD14 isolation kit
(Miltenyi Biotec, Bergisch Gladbach, Germany)
following the manufacturer’s instructions. Briefly,
PBMC were incubated in recommended buffer
with MACS
TM
CD14 MicroBeads for 15 min at
4
C, centrifugated and resuspended in buffer.
Later cells were passed through a positive selection
column. This step was done twice to obtain highly
purified CD14 positive cells.
After resuspension of monocytes in X-VIVO 15
they were seeded at defined cell densities described
at the results’ section (Standard concentration:
1.3 10
6
ml
1
) and placed in an incubator at
37
Cand5%CO
2
. Cytokines including rhuGM–CSF
and rhuIL-4 were added at day 0 (standard con-
centration: rhuGM–CSF (400 U ml
1
), rhuIL-4
(2000 U ml
1
)). All comparison experiments were
performed in 48 well plates (Greiner, Solingen,
Germany) and with optimized parameters up-
scaled in 75 cm
2
T-flasks (Greiner). After 6 days
of differentiation of monocytes into DC different
maturation cocktails were added: cocktail I (TNF-
(1000 U ml
1
), IL-1 (1000 U ml
1
), IL-6 (1000 U
ml
1
), PGE
2
(1 gml
1
, 0.003 M)) and cocktail II
(TNF- (1000 U ml
1
), PGE
2
(18 gml
1
,
122
0.051 M)). After 8 days the resulting suspension-
cells were harvested and analyzed as described.
Cell counting and viability
After harvesting of DC on day 8, counting and via-
bility determination was performed using a hemo-
cytometer with standard trypan blue dye exclusion
and a CASY 1 particle counting system (model TT,
Schaerfe System, Reutlingen, Germany).
Phenotyping and fluorescence activated cell
sorter analysis
For analysing of PBMC and DC populations we
used the following mAbs (all mAbs from Becton
Dickinson, Heidelberg, Germany): CD1a
(CyChrome), CD3 (CyChrome), CD4 (PE), CD8
(FITC), CD14 (FITC), CD16 (PE), CD19 (PE),
CD25 (FITC), CD40 (FITC), CD54 (PE), CD56
(FITC), CD80 (FITC), CD83 (PE), CD86 (FITC),
anti-HLA-A,B,C (FITC), anti-HLA-DR
(CyChrome, PE). Cells (2 10
5
per specimen)
were suspended in 90 l of ice-cold PBS and incu-
bated with 5 l of corresponding mAb for 30 min at
4
C. After staining cells were washed once with
ice-cold PBS and fixed in 200 l of 1% paraformal-
dehyde in PBS. FACS analysis was performed
using a FACSCalibur (Becton Dickinson) and
CellQuest 3.1 software (Becton Dickinson).
Cytokine enzyme-linked immunosorbent assay
(ELISA) kits
Cytokine ELISA kits for rhuGM–CSF (detec-
tion limit: 4.7 pg ml
1
), rhuIL-4 (detection limit:
7.8 pg ml
1
) and rhuIL-12p70 (detection limit: 7.8
pg ml
1
) were purchased from Becton Dickinson
(OptEIA human ELISA Set, BD PharMingen,
Heidelberg, Germany) and were used following
the manufacturer’s instructions. The readout of
the ELISA-plates was performed using a photo-
meter (Wallac Victor
2
, PerkinElmer Life Science,
Bad Wildbad, Germany) reader at 450 nm with a
correction at 570 nm.
Metabolic analysis
Osmolality was measured using a freezing-point
osmometer Osmomat 030 (Gonotec, Berlin,
Germany). Glucose (Ebio compact, Eppendorf,
Hamburg, Germany), lactate (YSI 1500L, Yellow
Springs Instruments, Yellow Springs, USA), gluta-
mine and glutamate (YSI 2700 select, Yellow
Springs Instruments, Yellow Springs, USA) were
quantified enzymatically using the indicated auto-
matic analyzer according to the manufacturers
instructions. Amino acids analysis was realized
using HPLC (Amino Quant 1090 AX, Hewlett
Packard, Waldbronn, Germany).
The MLR
DC were added to 5 10
5
allogeneic PBMC at a
ratio of 1 : 10 in 6 well plates and co-incubated for
4 days in RPMI 1640 supplemented with 10% FCS.
After 4 and 7 days 50% of the culture medium were
replaced and 100 U ml
1
rhuIL-2 were added.
After 8 days the proliferating T-cells were counted,
phenotyped and an Interferon- secretion assay
was performed.
Interferon- secretion assay
For determination of the portion of IFN- produc-
ing T-cells an IFN- Cytokine Secretion Assay
(Miltenyi Biotec, Bergisch Gladbach, Germany)
was used according to the manufacturer’s instruc-
tions (Manz et al. 1995).
Results
Cell enrichment and starting population of
monocytes
There are different methods of monocyte enrich-
ment currently discussed in literature. Due to the
availability of GMP (Good Manufacturing
Practice) quality immunomagnetic beads from
Miltenyi (CliniMACS
TM
) to isolate CD14
+
mono-
cytes (Dzionek et al. 2002), this method was used in
a laboratory setup with Mini- and Midi-MACS
TM
magnets and appropriate columns. This magnetic
cell sorting approach was highly effective in isolat-
ing CD14
+
monocytes from PBMC with a yield of
12.5 ± 4.8% (n ¼ 6) and a purity of 98% (data not
shown).
123
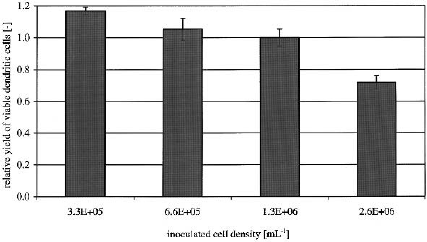
Influence of different cell densities on yield and
maturation of moDC
To setup and optimize a protocol for the genera-
tion of moDC we first investigated the influence
of different cell densities on the consumption and
accumulation of medium components such as glu-
cose, lactate, glutamine, glutamate and supplemen-
ted cytokines. In different experiments 3.3 10
5
,
6.6 10
5
, 1.3 10
6
and 2.6 10
6
monocytes ml
1
were inoculated in X-VIVO15 supplemented with
800 U ml
1
GM–CSF and 500 U ml
1
IL-4 and
incubated for 6 days without feeding. A maturation
cocktail consisting of TNF- (1000 U ml
1
), IL-1
(1000 U ml
1
), IL-6 (1000 U ml
1
) and PGE
2
(1 gml
1
) was then added and the cells incubated
for further 2 days.
After 6 days of cultivation partly non-adherent
DC could be observed which did not express CD83
a marker of mature DC (Zhou and Tedder 1995).
The maturation cocktail added for two additional
days induced the expression of CD83 and an
increase in CD80 and CD86 as well as HLA-DR.
These non-adherent DC with many motile veils
showed the typical pattern of mature moDC. The
size of the cells increased from an average of 10 m
(monocytes) to 16 m (moDC), which was deter-
mined by a CASY 1 particle counter.
The yield (as defined by size of cells, morphology
and surface antigen expression) of matured moDC
was similar for the cultures initially inoculated with
3.3 10
5
, 6.6 10
5
, 1.3 10
6
monocytes ml
1
and
about 25% lower for the highest inoculated cell
density (Figure 1). All cell densities except for the
2.6 10
6
ml
1
showed the typical mature DC
phenotype with high expression of HLA-A, B, C
(data not shown), HLA-DR, CD80, CD83
and CD86 (Figure 2). The DCs differentiated and
matured from 2.6 10
6
ml
1
expressed reduced
HLA-DR, CD80, CD83 and CD86 antigens. The
HLA-DR/CD80 and HLA-DR/CD83 dot plots
for 6.6 10
5
and 1.3 10
6
ml
1
showed two distinct
populations of DC: a population with higher HLA-
DR/CD80 and HLA-DR/CD83 expression and
a lower one, which may be caused by a different
maturation status. Especially for the HLA-DR/
CD80 dot plot for the cell density of 1.3 10
6
ml
1
this observation was significant. Lower HLA-DR
expression seems to correlate with a decrease
in CD80 surface antigen expression. The mean
fluorescent intensity as an indicator for the level
of expression of CD40 decreased about 50% with
the highest cell density (data not shown). We could
not observe any differences in expression of CD1a
(data not shown).
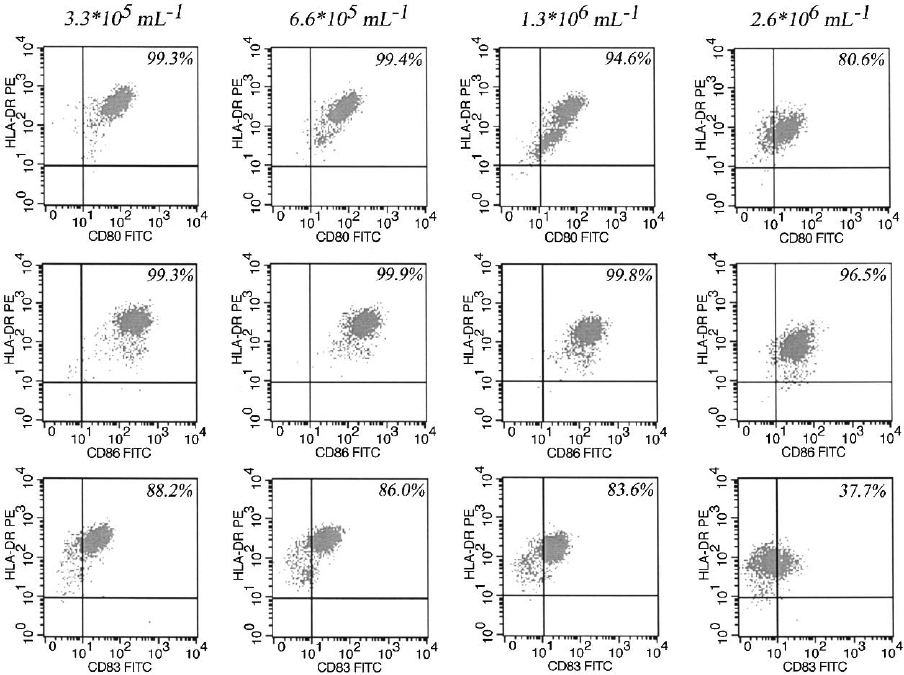
We also analyzed medium components and
found an increase in lactate concentration at the
highest cell density at 25 mmol l
1
(Figure 3),
which caused a decreased culture pH and might
be responsible for the lower yield. Accumulation of
metabolic products like lactate produces an acidic
environment and therefore inhibits proliferation
(Bohnenkamp and Noll 2002; Patel et al. 2000).
The glucose concentration in all experiments
remains above limiting levels. Amino acid analysis
demonstrated no limitation for glutamine and
serine. The highest glutamate concentration was
1.4 mmol l
1
.
An ELISA for GM–CSF and IL-4 showed no
limitation for GM–CSF (GM–CSF residual con-
tent: 3.3 10
5
ml
1
: 780 U ml
1
, 6.6 10
5
ml
1
:
720 U ml
1
, 1.3 10
6
ml
1
: 450 U ml
1
and 2.6
10
6
ml
1
: 500 U ml
1
, respectively) but IL-4 was
limiting (detection limit: 7.8 pg ml
1
) (data not
shown), which might explain the non-homogenous
DC population especially for the 6.6 10
5
and
1.3 10
6
ml
1
inoculated monocytes.
Due to a cost-effective utilization of the cultiva-
tion system in terms of highest yield of matured
moDC per volume, all further experiments were
Figure 1. Influence of different cell densities on yield after
generation of moDC. Monocytes were enriched via
immunomagnetic beads, inoculated at specified cell densities
and differentiated with 800 U ml
1
GM–CSF and 500 U ml
1
IL-4. For maturation a cytokine cocktail consisting of TNF-,
IL-1, IL-6 and PGE
2
was used. The data represent the mean
(± SD) of triplicates from a single donor. Similar data were
obtained with two other donors.
124
performed at a cell density of 1.3 10
6
ml
1
inoculated monocyte.
Optimization of GM–CSF and IL-4
concentrations
Based on the finding that no substrate feeding is
necessary for the generation of matured moDC at
a cell density of 1.3 10
6
ml
1
inoculated mono-
cytes, we investigated the influence of different
concentrations of GM–CSF and IL-4 on yield
and phenotype of the cells in order to get a homo-
genous moDC population. Monocytes differen-
tiate in the presence of GM–CSF and IL-4 to
immature DC but both cytokines are also
necessary for maturation, therefore suggesting
that both cytokines are not exhausted after 8 days
of culture.
We did several experiments to test the stability,
half-life and consumption of the mentioned cyto-
kines. The stability and half-life test was estab-
lished utilizing triplicates of T-flasks we also used
for the generation of DC. We inoculated 800 U ml
1
GM–CSF and 500 U ml
1
IL-4 respectively in
X-VIVO 15 and incubated at 37
C and 5% CO
2
for 8 days. Samples were taken every [2
nd
] day,
frozen and after collection thawed for quantifica-
tion of GM–CSF and IL-4. The GM–CSF used
(Leucomax
TM
) showed no decrease of concentra-
tion after 8 days (data not shown). For the IL-4
Figure 2. Phenotype of moDC generated with in figure 1 specified cultivation parameters. HLA-DR/CD80, HLA-DR/CD86 and HLA-
DR/CD83 dot plots are shown for different cell densities (3.3 10
5
, 6.6 10
5
, 1.3 10
6
and 2.6 10
6
ml
1
respectively). Decreased
CD80, CD83 and CD86 was expressed for the highest cell density, HLA-DR/CD80 and HLA-DR/CD83 dot plots for 6.6 10
5
and
1.3 10
6
ml
1
featured a higher and a lower expressing population. The data shown are from one representative experiment out of three
performed.
125
(R&D) we could demonstrate that 80% of the cyto-
kine adhered to the surface of the flask after 2 h.
The remaining IL-4 concentration did not show
any further decay (data not shown). Therefore,
the monocytes were inoculated first and then the
cytokines were added to ensure that enough IL-4
remained in solution (data not shown).
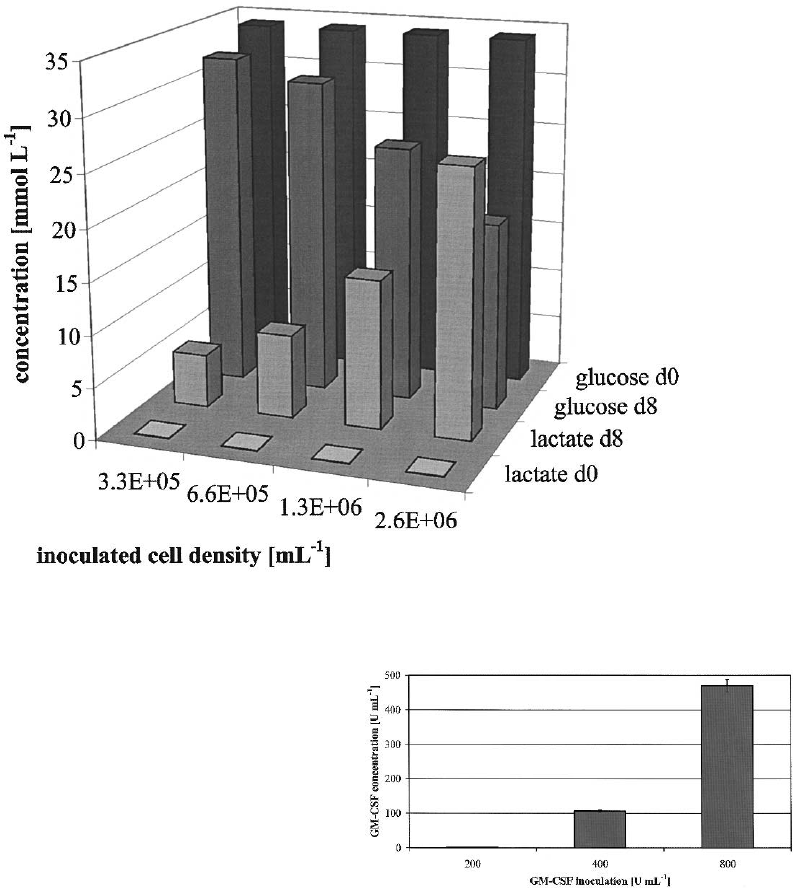
The next step was to investigate the consumption
of GM–CSF and IL-4. Therefore we started with
200, 400 and 800 U ml
1
GM–CSF while IL-4 was
inoculated at 500 U ml
1
. The 200 U ml
1
were
exhausted completely while in the other two
experiments about 300 U ml
1
were consumed
(296 U ml
1
and 320 U ml
1
, respectively)
(Figure 4). The yield and phenotype was compar-
able to previous experiments, see Figure 2, 1.3
10
6
ml
1
inoculated monocytes. The 200 U ml
1
GM–CSF concentration resulted in the same
number of DCs but in a lower expression of
CD80, CD83 and CD86 (data not shown).
We then determined which concentration of IL-4
resulted in a homogenous populations of matured
moDC. We added different IL-4 concentrations
(500, 1000 and 2000 U ml
1
respectively) with a
constant concentration of 800 U ml
1
GM–CSF to
alter only one parameter. The IL-4 ELISA
indicated that only at an initial concentration of
2000 U ml
1
IL-4 was the not limiting, as we could
still measure 25 U ml
1
after 8 days of cultivation
(data not shown) and phenotypic analysis showed
Figure 4. GM–CSF consumption of cultivated cells after 8 days.
Data shown are the mean (± SD) of triplicate cultures from one
representative experiment of three performed.
Figure 3. Glucose and lactate analysis on day 0 (start concentration) and on day 8 (after generation of moDC). Shown is one
representative experiment out of three.
126
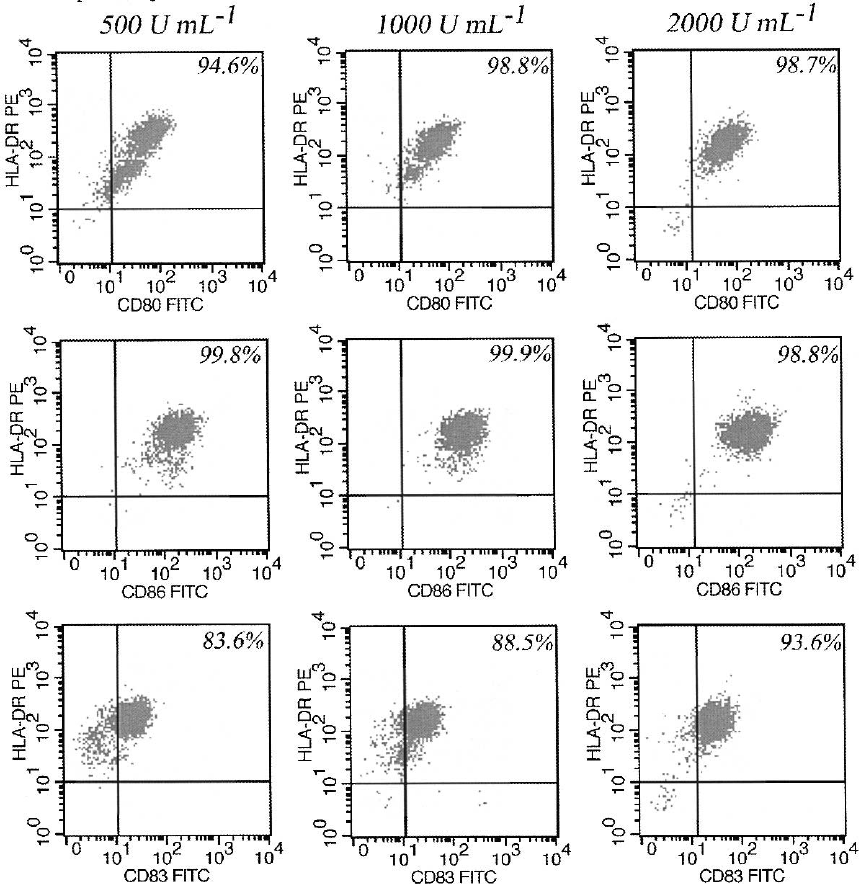
a homogenous population of matured moDC only
for the highest IL-4 concentration (Figure 5).
However, the number of DCs was similar for all
cytokine concentrations.
The data demonstrated that800 U ml
1
GM–CSF
and 2000 U ml
1
IL-4 resulted in homogenously
matured moDC. We also could show that
400 U ml
1
GM-CSF is sufficient for the genera-
tion of mature moDC without feeding.
Influence of different maturation cocktails
In previous experiments for maturation of moDC a
cocktail consisting of TNF-,IL-1, IL-6 and
Figure 5. Phenotype of moDC generated with different IL-4 concentrations (500, 1000 and 2000 U ml
1
). Only moDC generated with
2000 U ml
1
demonstrated a homogenous population of fully matured dendritic cells. The data shown are from one representative
experiment of three performed.
127
PGE
2
was used. In the following experiments, we
examined the feasibility to simplify this maturation
cocktail while obtaining the same yield, viability,
phenotype and distinct functional capacity of DC.
We used 400 U ml
1
GM–CSF, 2000 U ml
1
IL-4
and inoculated a cell density of 1.3 10
6
ml
1
to
differentiate monocytes to dendritic cells.
Therefore, we compared two maturation cock-
tails: Cocktail I composed of 1000 U ml
1
TNF-,
1000 U ml
1
IL-1, 1000 U ml
1
IL-6 and 1-gml
1
PGE
2
and Cocktail II consisted of 1000 U ml
1
TNF- and 18 gml
1
PGE
2
. We found in 12
experiments similar results in yield, viability and
phenotype for both cocktails (data not shown).
A consequence of maturation is usually the
secretion of inflammatory cytokines by moDC
(Sallusto and Lanzavecchia 1999). However, it
has been demonstrated that PGE
2
induces the
final maturation of IL-12p70 deficient DC
(Kalinski et al. 1998). To investigate the amount
of bioreactive IL-12p70, we analyzed by ELISA the
cell culture supernatant of moDC after 2 days of
stimulation with the respective cytokine cocktail.
With both stimuli no detectable level of IL-12p70
was produced at any time point (data not shown).
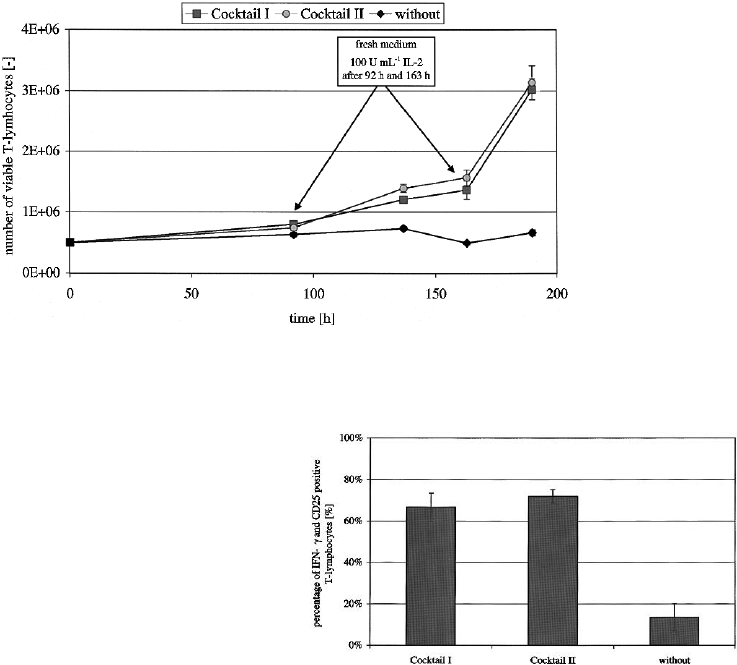
Mixed lymphocyte reaction
The allostimulatory capacity of matured moDC
was tested using a DC T-cell ratio of 1 : 10. After
an induction phase cells were fed twice at 92 h and
163 h by replacement of 50% of the medium and
adding of 100 U ml
1
IL-2. Figure 6 illustrates the
potent stimulatory capacity produced by moDC
samples.
Testing of the supernatant of the MLR after
4 days for levels of IL-12p70 showed no detectable
amount of cytokine. Since PGE
2
suppressed
IL-12p70 production in matured moDC, we inves-
tigated whether a typical T
H
1orT
H
2 cytokine
pattern was being induced. After 190 h of cultiva-
tion and proliferation of T-lymphocytes the super-
natant was tested for secreted IFN-. Either T-cells
stimulated by moDC matured with cocktail II or I
were highly IFN- and CD25 positive (Figure 7).
ELISA for IL-4 showed no level of cytokine (data
not shown). These data suggest that T-lymphocytes
stimulated by moDC matured by either cytokine
cocktails were polarized towards T
H
1-type.
Figure 6. Allostimulatory capacity for PBMC from healthy donors. MoDC matured with different maturation cocktails (cocktail I:
TNF-, IL-1, IL-6 and PGE
2
; cocktail II: TNF-, PGE
2
) induced a similar stimulatory capacity in the allogeneic MLR. Shown are
mean values (± SD) from three experiments in triplicates.
Figure 7. Phenotype of PBMC after MLR. Either cocktail
I and II induced IFN- producing T-cells which were also
CD25 (IL-2 -chain) positive. Results are expressed as mean
(± SD) from three experiments in triplicates.
128
Generation of moDC with optimized parameters
Based on the optimized parameters, generation of
moDC was carried out in 75 cm
2
T-flasks.
Monocytes were inoculated at a cell density of
1.3 10
6
ml
1
and differentiated with 400 U ml
1
GM–CSF and 2000 U ml
1
IL-4. On day 6
1000 U ml
1
TNF- and 18 gml
1
PGE
2
were
added to mature the cells and on day 8 the cells
were harvested.
No difference was observed regardless of using
48 well plates or 75 cm
2
T-flasks. The mean yield as
calculated from inoculated CD14+ monocytes was
31 ± 6% with a mean viability of 93 ± 2% (n ¼ 4).
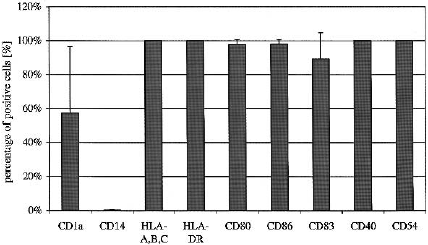
Figure 8 illustrates the mean percentage of positive
DC for important surface antigens like HLA-DR,
HLA-A,B,C, costimulatory molecules like CD40,
CD80 and CD86, the intercellular adhesion mole-
cule (ICAM-1) CD54 and the standard maturation
marker CD83.
Discussion
The aim of this study was to determine optimal
conditions for the generation of moDC by devel-
oping a standardized, easy to use and reproducible
protocol. We accomplished within this study a non-
feeding protocol in serum-free conditions that is
suitable for clinical use. Culture of enriched
monocytes using serum-free conditions for 6 days
resulted in immature moDC with low levels of
CD83. Final maturation was induced for
additional 2 days by pro-inflammatory cytokines
TNF-,IL-1 and IL-6 supported by PGE
2
(Jonuleit et al. 1997) or TNF- and PGE
2
(Kalinski et al. 1998) alone, both of which resulted
in high levels of HLA-DR, CD80, CD83, CD86,
CD40 and CD54.
Using this protocol we could obtain moDC with
a yield of 31 ± 6% and a viability of 93 ± 2%. We
demonstrated that feeding the cells is not necessary
and that it is sufficient to only add 400 U ml
1
GM–CSF and 2000 U ml
1
IL-4 at the beginning
of the cultivation. Dietz et al. (2000), who gener-
ated moDC using MACS Micro-beads enriched
CD14
+
monocytes in X-VIVO 15, 1% human AB
serum, 800 U ml
1
GM–CSF and 1000 U ml
1
IL-4
fed the cells every 3rd day by replacing of one-third
with fresh medium containing 1600 U ml
1
GM–
CSF and 1000 U ml
1
IL-4 obtained a yield
between 11.4% and 31.2%. Berger et al. (2002)
reported a moDC yield of 19.9 ± 9.6% with a
viability of 94.0% ± 12.0%, using the adherent
fraction of PBMC: the cells were cultivated in
RPMI 1640 supplemented with 1% autologous,
heat-inactivated human plasma, 800 U ml
1
GM–CSF and 500 U ml
1
IL-4 and fed twice on
day 3 and 5 with additional medium, 800 U ml
1
GM–CSF and 500 U ml
1
IL-4.
Our data suggest that under serum-free con-
ditions the cytokine-cocktails consisting of either
TNF-,IL-1, IL-6 and PGE
2
or TNF- and
PGE
2
, a simplified cocktail with just two compo-
nents, are sufficient to induce the final maturation
of immature moDC into mature, homogenous
immunostimulatory DC. Stimulation of allogeneic
na
€
ve T-cells in MLR led to high proliferation with
either cytokine cocktail. Production of IFN- was
significantly induced, while no effect on the pro-
duction of IL-4 or IL-12p70 was seen. These find-
ings are in agreement with Jonuleit et al. (1997),
who demonstrated that addition of PGE
2
to a
cocktail of TNF-, IL-1 and IL-6 led to higher
IFN- production in allogeneic primary stimula-
tion. They also observed, that neither CD4
+
nor
CD8
+
T-cells produced IL-4 or IL-10, indicating
that these moDC could not support the develop-
ment of type-2 T-cells. Two studies showed
recently that PGE
2
regulates the migratory capa-
city of moDC (Luft et al. 2002; Scandella et al.
2002) and that these migratory-type DC produce
lower level of cytokines (including IL-12p70) and
induce IFN- production of T-cells in MLR. These
Figure 8. Phenotypic analysis of mature moDC generated with
the standardized protocol. Shown are results of surface antigen
expression as indicated as mean (± SD) from four independent
experiments.
129
conclusions were confirmed by several clinical
investigations using moDC matured with the
cytokine-cocktails utilized in this study (TNF-,
IL-1, IL-6, PGE
2
), which induced more potent
T-cell immune responses in undergoing immu-
notherapy patients (Schuler-Thurner et al. 2002;
Dhodapkar et al. 2001). These findings were in
contrast to Kalinski et al. (1998) who reported
that DC matured in the additional presence of
PGE
2
bias na
€
ve Th cell development toward the
Th2. However, this may be caused by different
cultivation parameters including FCS in the
cultivation setup.
In conclusion, we have shown that we could
further simplify the generation of fully mature
moDC while maintaining their high stimulatory
capacity. Although for the clinical application of
DCs the culture will need to take place in a fully
enclosed system, for instance cell bags (Guyre et al.
2002). Because of the simplicity of the protocol
described here the transfer to such a system should
be easily achieved.
Acknowledgements
The authors gratefully acknowledge Professor
Dr C. Wandrey for his support, B. Schwartzkopff
for performing medium analysis and cytokine
ELISA and C. Herfurth for amino acids analysis.
We also like to thank Dr Joy Burchell for critical
comments. This work was funded partly by the
European Commission (5th frame project, number:
QLK3-2002-01980).
References
Banchereau J. and Steinman R.M. 1998. Dendritic cells and the
control of immunity. Nature 392: 245–252.
Bender A., Sapp M., Schuler G., Steinman R.M. and Bhardwaj N.
1996. Improved methods for the generation of dendritic cells
from nonproliferating progenitors in human blood.
J. Immunol. Meth. 196: 121–135.
Berger T., Feuerstein B., Strasser E., Hirsch U., Schreiner D.,
Schuler G. and Schuler-Thurner B. 2002. Large-scale genera-
tion of mature monocyte-derived dendritic cells for clinical
application in cell factories. J. Immunol. Meth. 268: 131–140.
Bernard J., Ittelet D., Christoph A., Potron G., Adjizian J.C.,
Kochman S. and Lopez M. 1998. Adherent-free generation of
functional dendritic cells from purified blood monocytes in
view of potential clinical use. Hematol. Cell Ther. 40: 17–26.
Bohnenkamp H.R. and Noll T. 2002. Bioprocess development
for the cultivation of human T-lymphocytes in a clinical scale.
Cytotechnology 38: 135–145.
Caux C., Dezutter-Dambuyant C., Schmitt D. and Banchereau J.
1992. GM-CSF and TNF-alpha cooperate in the generation
of dendritic Langerhans cells. Nature 360: 258–261.
Costello R.T., Gastaut J.A. and Olive D. 1999. Tumor escape
from immune surveillance. Arch. Immunol. Ther. Exp. 47:
83–88.
Dhodapkar M.V., Steinman R.M., Krasovsky J., Munz C. and
Bhardwaj N. 2001. Antigen-specific inhibition of effector T
cell function in humans after injection of immature dendritic
cells. J. Exp. Med. 193: 233–238.
Dietz A.B.,Bulur P.A., Erickson M.R., Wettstein P.J., Litzow M.R.,
Wyatt W.A., Dewald G.W., Tefferi A., Pankratz V.S. and
Vuk-Pavlovic S. 2000. Optimizing preparation of normal
dendritic cells and bcr-abl+ mature dendritic cells
derived from immunomagnetically purified CD14+ cells.
J. Hematother. Stem Cell Res. 9: 95–101.
Dzionek A., Piechaczek C., Campbell J., Zysk M., Winkels G.,
Huppert V. and Schmitz J. 2002. Clinical-scale magnetic
sorting and multiparamter analysis of dendritic cells aubsets
and precursers. In: 7th International Symposium on Dendritic
Cells, Bamberg.
Fong L., Hou Y., Rivas A., Benike C., Yuen A., Fisher G.A.,
Davis M.M. and Engleman E.G. 2001. Altered peptide ligand
vaccination with Flt3 ligand expanded dendritic cells for
tumor immunotherapy. Proc. Natl. Acad. Sci. USA 98:
8809–8814.
Goxe B., Latour N., Chokri M., Abastado J.P. and Salcedo M.
2000. Simplified method to generate large quantities of den-
dritic cells suitable for clinical applications. Immunol. Invest.
29: 319–336.
Guyre C.A., Fisher J.L., Waugh M.G., Wallace P.K., Tretter
C.G., Ernstoff M.S. and Barth R.J. 2002. Advantages of
hydrophobic culture bags over flasks for the generation
of monocyte-derived dendritic cells for clinical applications.
J. Immunol. Meth. 262: 85–94.
Jonuleit H., Kuhn U., Muller G., Steinbrink K., Paragnik L.,
Schmitt E., Knop J. and Enk A.H. 1997. Pro-inflammatory
cytokines and prostaglandins induce maturation of potent
immunostimulatory dendritic cells under fetal calf serum-
free conditions. Eur. J. Immunol. 27: 3135–3142.
Kalinski P., Schuitemaker J.H., Hilkens C.M. and Kapsenberg
M.L. 1998. Prostaglandin E2 induces the final maturation of
IL-12-deficient CD1a+CD83+ dendritic cells: The levels of
IL-12 are determined during the final dendritic cell matura-
tion and are resistant to further modulation. J. Immunol. 161:
2804–2809.
Kikuchi T., Akasaki Y., Irie M., Homma S., Abe T. and Ohno T.
2001. Results of a phase I clinical trial of vaccination of
glioma patients with fusions of dendritic and glioma cells.
Cancer Immunol. Immunother. 50: 337–344.
Kobayashi T., Shinohara H., Toyoda M., Iwamoto S. and
Tanigawa N. 2001. Regression of lymph node metastases by
immunotherapy using autologous breast tumor-lysate pulsed
dendritic cells: Report of a case. Surg. Today 31: 513–516.
Kugler A., Stuhler G., Walden P., Zoller G., Zobywalski A.,
Brossart P., Trefzer U., Ullrich S., Muller C.A., Becker V.,
Gross A.J., Hemmerlein B., Kanz L., Muller G.A. and
130
Ringert R.H. 2000. Regression of human metastatic renal cell
carcinoma after vaccination with tumor cell-dendritic cell
hybrids. Nat. Med. 6: 332–336.
Luft T., Jefford M., Luetjens P., Toy T., Hochrein H.,
Masterman K.A., Maliszewski C., Shortman K., Cebon J.
and Maraskovski E. 2002. Functionally distinct dendritic
cell DC populations induced by physiologic stimuli: prosta-
glandin E2 regulates the migratory capacity of specific DC
subsets. Blood 100: 1362–1372.
Mackensen A., Herbst B., Chen J.L., Kohler G., Noppen C.,
Herr W., Spagnoli G.C., Cerundolo V. and Lindemann A.
2000. Phase I study in melanoma patients of a vaccine
with peptide-pulsed dendritic cells generated in vitro from
CD34(+) hematopoietic progenitor cells. Int. J. Cancer 86:
385–392.
Manz R., Assenmacher M., Pfluger E., Miltenyi S. and
Radbruch A. 1995. Analysis and sorting of live cells according
to secreted molecules, relocated to a cell-surface affinity
matrix. Proc. Natl. Acad. Sci. USA 92: 1921–1925.
Nestle F.O., Alijagic S., Gilliet M., Sun Y., Grabbe S.,
Dummer R., Burg G. and Schadendorf D. 1998.
Vaccination of melanoma patients with peptide- or tumor
lysate-pulsed dendritic cells. Nat. Med. 4: 328–332.
O’Garra A. and Arai N. 2000. The molecular basis of T helper 1
and T helper 2 cell differentiation. Trends Cell Biol. 10:
542–550.
Patel S.D., Papoutsakis E.T., Winter J.N. and Miller W.M.
2000. The lactate issue revisited: Novel feeding protocols
to examine inhibition of cell proliferation and glucose meta-
bolism in hematopoietic cell cultures. Biotechnol. Prog. 16:
885–892.
Pickl W.F., Majdic O., Kohl P., Stockl J., Riedl E., Scheinecker C.,
Bello-Fernandez C. and Knapp W. 1996. Molecular and
functional characteristics of dendritic cells generated
from highly purified CD14+ peripheral blood monocytes.
J. Immunol. 157: 3850–3859.
Romani N., Gruner S., Brang D., Kampgen E., Lenz A.,
Trockenbacher B., Konwalinka G., Fritsch P.O., Steinman
R.M. and Schuler G. 1994. Proliferating dendritic cell
progenitors in human blood. J. Exp. Med. 180: 83–93.
Romani N., Reider D., Heuer M., Ebner S., Kampgen E.,
Eibl B., Niederwieser D. and Schuler G. 1996. Generation of
mature dendritic cells from human blood. An improved
method with special regard to clinical applicability.
J. Immunol. Meth. 196: 137–151.
Sallusto F. and Lanzavecchia A. 1994. Efficient presentation of
soluble antigen by cultured human dendritic cells is main-
tained by granulocyte/macrophage colony-stimulating factor
plus interleukin 4 and downregulated by tumor necrosis factor
alpha. J. Exp. Med. 179: 1109–1118.
Sallusto F. and Lanzavecchia A. 1999. Mobilizing dendritic
cells for tolerance, priming, and chronic inflammation.
J. Exp. Med. 189: 611–614.
Scandella E., Men Y., Gillessen S., Forster R. and Groetrp M.
2002. Prostaglandin E2 is a key factor for CCR7 surface
expression and migartion of monocyte-derived dendritic
cells. Blood 100: 1354–1361.
Schuler-Thurner B., Schultz E.S., Berger T.G., Weinlich G.,
Ebner S., Woerl P., Bender A., Feuerstein B., Fritsch P.O.,
Romani N. and Schuler G. 2002. Rapid induction of tumor-
specific type 1 helper cells in metastatic melanoma patients
by vaccination with mature, cryopreserved, peptide-
loaded monocyte-derived dendritic cells. J. Exp. Med. 195:
1279–1288.
Thurner B., Haendle I., Roder C., Dieckmann D.,
Keikavoussi P., Jonuleit H., Bender A., Maczek C.,
Schreiner D., von den Driesch P., Brocker E.B.,
Steinman R.M., Enk A., Kampgen E. and Schuler G. 1999a.
Vaccination with mage-3A1 peptide-pulsed mature, mono-
cyte-derived dendritic cells expands specific cytotoxic T cells
and induces regression of some metastases in advanced stage
IV melanoma. J. Exp. Med. 190: 1669–1678.
Thurner B., Roder C., Dieckmann D., Heuer M., Kruse M.,
Glaser A., Keikavoussi P., Kampgen E., Bender A. and
Schuler G. 1999b. Generation of large numbers of fully
mature and stable dendritic cells from leukapheresis products
for clinical application. J. Immunol. Meth. 223: 1–15.
Zhou L.J. and Tedder T.F. 1995. Human blood dendritic cells
selectively express CD83, a member of the immunoglobulin
superfamily. J. Immunol. 154: 3821–3835.
131
