
From monocyte isolation to DC characterization
Generation of Mo-DCs
Introduction
As professional antigen-presenting cells, dendritic
cells (DCs) are a cornerstone of the adaptive immune
system. Various clinical trials have shown the great
immunotherapeutic potential of DC-based vaccines in the
treatment of tumors.¹
Immature DCs residing in peripheral tissues, including
blood, have the capacity to take up tumor-associated
antigens, for example. Following maturation, DCs
up-regulate their antigen processing machinery, express
a variety of cell surface markers that are involved in the
formation of immunological synapse, and migrate from the
periphery to draining lymph nodes where they present the
antigen to T cells². Both antigen presentation and triggering
of CD28 on T cells by the DC costimulatory markers, CD80
and CD86³, are required to induce an antigen-specific
response. Upon stimulation T cells up-regulate the
expression of CD40 ligand (CD40L) and proliferate to
counteract the disease that is associated with the particular
pathogenic antigen. Binding of CD40L on activated T helper
(T
h) cells to CD40 on mature DCs provides a feedback signal
that leads to the secretion of IL-12 by DCs, which in turn
drives T cell polarization towards the T
h1 lineage and the
antitumor response mediated by cytotoxic T cells.
Large numbers of DCs for basic research and
immunotherapies can be generated in vitro from
monocytes
5,6
. Differentiation/maturation protocols have
been described by various groups
7–10
. To obtain functional
monocyte-derived DCs (Mo-DCs) that possess the distinct
phenotype and function of natural DCs, it is crucial to
follow reliable procedures using high-quality reagents.
This application note describes all the steps for the
generation of Mo-DCs and their phenotypic and functional
characterization.
Material and methods
Isolation of monocytes
CD14
+
monocytes were isolated from peripheral blood
mononuclear cells (PBMCs) from healthy donors by
MACS® Technology using CD14 MicroBeads (Miltenyi Biotec).
Purity and recovery were determined by labeling cells with
CD14-FITC antibodies and subsequent flow cytometry
analysis on a MACSQuant® Analyzer 10 (Miltenyi Biotec).
Cell viability was determined according to propidium
iodide (PI) staining.
Differentiation and maturation of monocyte-derived
dendritic cells (Mo-DCs)
The generation of Mo-DCs can be achieved by using various
protocol formats, manually and automatically, at various
scales. Here we describe the procedure for 6-well plates as
an example. On day 0, isolated monocytes (3×10 cells) are
cultured in 3 mL of complete medium (RPMI 1640, 2 mM
L-glutamine, 1% autologous plasma), supplemented
7,8
with
250 IU/mL IL-4 and 800 IU/mL GM-CSF, and incubated at
37°C and 5% CO. On day 2, a volume of 1.5 mL is removed
from the culture and centrifuged. Cells are resuspended in
1.5 mL of complete medium supplemented with the 2-fold
concentration of IL-4 and GM-CSF and put back into the
original culture. On day 6, a volume of 1.5 mL is removed
from the culture and centrifuged. Cells are resuspended in
1.5 mL medium supplemented
9,10
with 2000 IU/mL IL-6, 400
IU/mL IL-1β, 2000 IU/mL TNF-α, and 2 µg/mL PGE, put back
into the original culture, and cultured for another 24 hours.
On day 7, viability, yield, and absolute cell count of mature
Mo-DCs (mMo-DCs) is determined by flow cytometry via
light scatter signals and PI fluorescence. For the gating
strategy see figure 1.
For all experiments shown in this application note, Mo-DCs
were generated using cytokines from Miltenyi Biotec.
Analysis of cell morphology
Monocytes as well as immature Mo-DCs (imMo-DCs) and
mMo-DCs were placed in a 6-well plate and allowed to
sediment. Images of the cells were captured using a light
microscope with phase-contrast at a 400× magnification.
Immunophenotyping of monocytes and Mo-DCs
by flow cytometry
To analyze cell surface expression of various maturation
markers, co-stimulatory molecules, and receptors for
chemokines and antigens, the monocytes, imMo-DCs,
and mMo-DCs were labeled with specific monoclonal
Generation of Mo-DCs | June 2016 1/8 Copyright © 2016 Miltenyi Biotec GmbH. All rights reserved.

antibodies (table 1). Non-specific antibody binding was
assessed using appropriate isotype controls. The mean
fluorescence intensity (MFI) of the respective markers was
determined by flow cytometry on a MACSQuant Analyzer
10 using the MACSQuantify™ Software (Miltenyi Biotec).
Cell debris and dead cells were excluded from the analysis
based on scatter signals and PI fluorescence. All antibodies
and Propidium Iodide Solution were from Miltenyi Biotec.
Cell surface antigen Clone Fluorochrome
CD1a HI149 PE
CD14 TÜK4 FITC
CD25 3G10 APC
CD HB APC
CD REA PE
CD REA PE
CD REA APC
CD FM PE
CD DCN APC
CD (CCR) REA APC
CD (DC-SIGN) REA APC
HLA-DR AC FITC
HLA-ABC REA FITC
Table : Antibodies used for monocyte and Mo-DC
immunophenotyping. All antibodies were from Miltenyi Biotec.
Antigen uptake capacity
To assess the pinocytosis capacity, 1×10 monocytes,
imMo-DCs, or mMo-DCs were incubated with FITC-labeled
dextran (1 mg/mL) in complete medium (RPMI, 2 mM
L-glutamine, 1% autologous plasma) for 5, 10, 20, 30, and
60 minutes at 37 °C. To check for non-specific binding of
FITC-dextran to the cell surface, a control sample was kept
on ice for 60 min. After 60 min, all samples were washed
twice (centrifugation at 300×g, 5 min, 4 °C) with ice-cold
PBS supplemented with 1% fetal calf serum (FCS) and finally
suspended in PBS supplemented with 0.5% BSA. Samples
were kept on ice until flow cytometry analysis. Uptake of
the FITC-dextran was determined by measuring the mean
fluorescence intensity (MFI) of FITC by flow cytometry.
Dead cells were excluded from the analysis
by PI fluorescence. Specific uptake of FITC-dextran was
calculated by subtracting the MFI of the control sample
that was incubated on ice from the MFI of the samples
incubated at 37 °C.
Migratory capacity of mMo-DCs
CCR7-dependent migration of mMo-DCs towards CCL19 was
tested in 24-well Transwell® Plates (Corning; pore size 5 µm).
The mMo-DCs were resuspended in RPMI 1640 with 10%
FCS at a density of 5×10 cells/mL, and 200 µL were placed
in the upper compartment of a Transwell Plate. The lower
compartment was filled with 600 µL of complete medium
supplemented with Human CCL19 (MIP-3β) (Miltenyi Biotec)
at different concentrations. After 3 h the cells contained in
the lower compartment were harvested and counted on a
MACSQuant® Analyzer 10.
Isolation of human naive CD4
+
T cells for mixed
lymphocyte reaction (MLR)
Naive allogeneic CD4
+
T cells were isolated from PBMCs
using the Naive CD4
+
T Cell Isolation Kit II, human
(Miltenyi Biotec) according to the instructions provided in
the data sheet. Purity of the isolated cells was determined
by labeling with i) CD4-PE or ii) CD45RO-APC and CD45RA-
PE antibodies (all from Miltenyi Biotec) and subsequent
analysis by flow cytometry using the MACSQuant
Analyzer10.
Assessment of the T cell priming capacity
of Mo-DCs in MLR
The capacity of Mo-DCs to induce proliferation of naive
T cells was measured in MLR. To this end, 5×10 purified
naive CD4
+
T cells were suspended in 400 µL PBS, and
labeled with 100 µL of a 10 µM CellTrace™ Violet solution
(Life Technologies®) for 5 min at RT. Subsequently, the cells
were washed once with 1 mL FCS and three times with
2 mL MLR medium (RPMI 1640, 2 mM L-glutamine, non-
essential amino acids, 0.1 mM sodium pyruvate, 5% human
AB serum). Finally, the CD4
+
T cells were suspended in MLR
medium at a density of 5×10 cells/mL.
Monocytes, imMo-DCs, and mMo-DCs were suspended
in MLR medium at a density of 1×10 cells/mL and
serially diluted according to table 2. The different cell
dilutions (100µL per well) were placed in a 96-well plate.
Subsequently, the labeled T cells (100 µL per well) were
added and cultured for 7 days at 37 °C, 5% CO.
Proliferation of CD4
+
T cells was determined by measuring
the fluorescence of CellTrace Violet by flow cytometry.
Cell debris and dead cells were excluded from the analysis
by scatter signals and PI fluorescence.
Mo-DC/monocyte
density (cells/mL)
Number of Mo-DCs/
monocytes per well
Ratio of Mo-DCs/
monocytes to
Tcells
2.5×10 25,000 1:2
1.25×10 12,500 1:4
6.25×10 6,250 1:8
.×10 , :
.× , :
.׳ :
Table : Serial dilution of Mo-DCs/monocytes for MLR assay.
The number of naive T cells was constant at ,.
Quantitation of IL-12 and IL-10 secreted
by mMo-DCs after stimulation with CD40L
The mMo-DCs were suspended in medium (RPMI, 2 mM
L-glutamine, 1% autologous plasma) at a density of 1×10
cells/mL, and 5×10 cells were used for the assay performed
in 96-well plates.
To measure the secretion of IL-12p70 and IL-10, mMo-DCs
were stimulated with soluble Human CD40-Ligand (16µg/
mL; Miltenyi Biotec) or in a co-culture with J558L cells
expressing CD40L (7×10, 14×10, or 28×10 cells/well) for
comparison. Mo-DCs were stimulated for 24 h at 37 °C, 5%
CO. Subsequently, the cell supernatants were collected
and centrifuged (300×g; 10 min) to remove any cells. The
concentrations of IL-12p70 and IL-10 were determined by
Generation of Mo-DCs | June 2016 2/8 Copyright © 2016 Miltenyi Biotec GmbH. All rights reserved.
specific ELISAs (eBioscience). The CD40L-expressing J558L
cell line was kindly provided by Professor Marina Cella
(Washington University, St. Louis, MO, USA)
Results
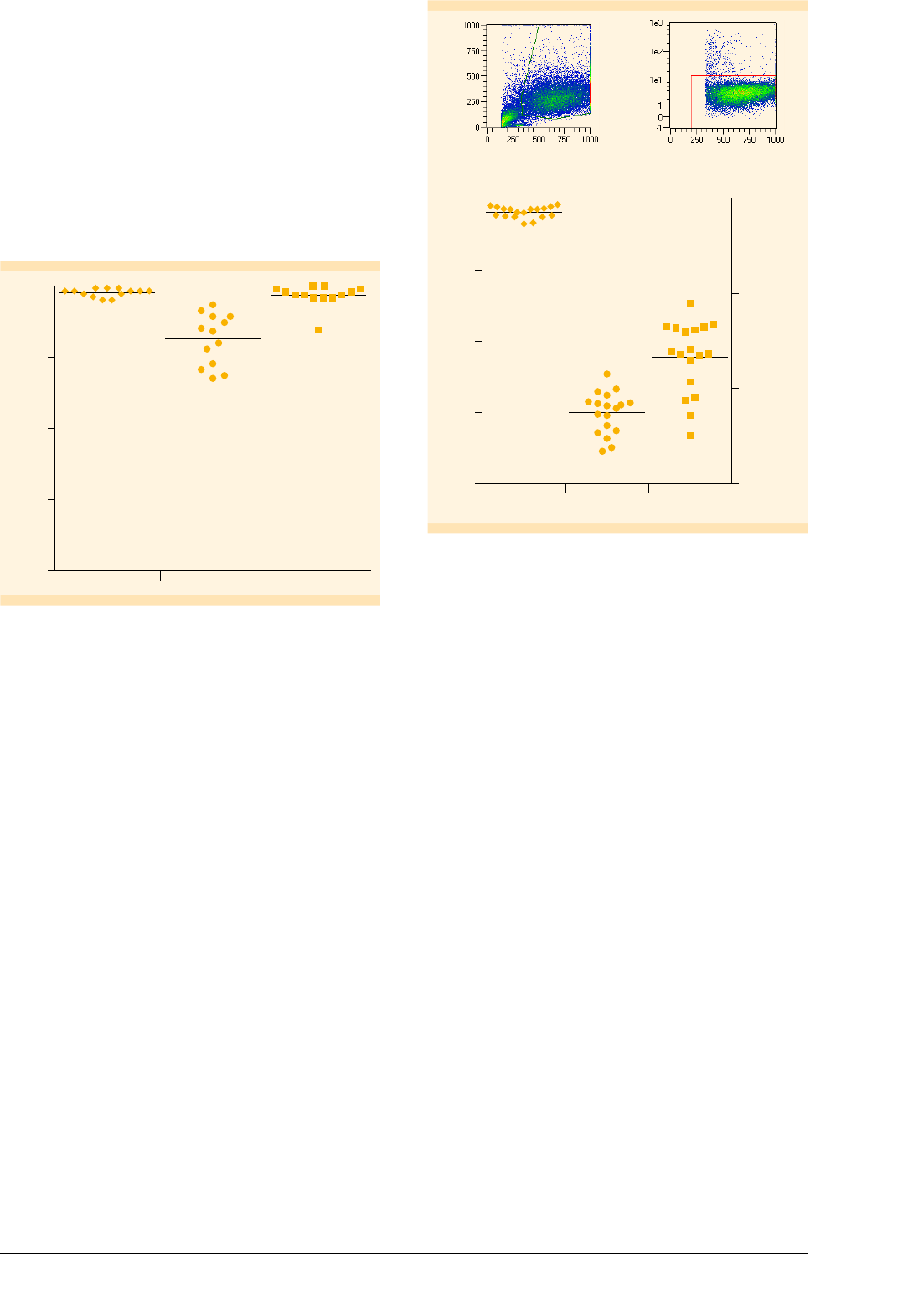
Isolation of monocytes to high purities
CD14
+
monocytes were isolated from PBMCs by MACS
Technology to a purity of 96.8±3.8%. The proportion
of CD14
+
cells that were recovered from the PBMC
fraction amounted to 81.3±8.8%%, and the viability
was 97.5±1.4% (fig. 1).
Percentage
100
75
50
25
0
Viability PurityRecovery
97.5 +/– 1.4%
81.3 +/– 8.8%
96.8 +/– 3.8%
Figure : Isolation of CD
+
monocytes. Purity, recovery (i.e.,
percentage of CD
+
cells in the purified fraction in relation to the
original fraction), and viability of enriched CD
+
cells are shown.
Cells were labeled with a CD-FITC antibody and analyzed by
flow cytometry.
Differentiation of monocytes into mature Mo-DCs
Purified monocytes were used to generate Mo-DCs
according to the protocol for 6-well plates. On day 7, cells
were analyzed by flow cytometry using the gating strategy
depicted in figure 2. The viability of mMo-DCs, evaluated
by excluding PI
+
dead cells, amounted to 95.4±2.4%.
The recovery of mMo-DCs was 23%, calculated based on
the number of originally seeded monocytes (fig. 2).
Side scatter
Forward scatter
97.7%
PI
Forward scatter
Figure : Generation of Mo-DCs. Viability, yield (i.e., mMo-DC count
in relation to initial monocyte count), and total count of mMo-DCs
generated by using the protocol for -well plates (n= ). Data were
obtained by flow cytometry on the MACSQuant Analyzer following
the gating strategy shown: First cells were gated according to their
scatter properties. Subsequently, dead cells were excluded from the
analysis by PI fluorescence.
Mo-DCs assume the characteristic DC morphology
during differentiation
Dendritic cells have a distinct morphology characterized
by many cellular processes. To evaluate whether monocytes
assume this morphology during differentiation and
maturation to Mo-DCs, we analyzed the cells on days
0, 6, and 7 of cell culture, both by microscopy and flow
cytometry. On day 0, the cells had a spherical shape,
which is normal for monocytes. After 6 days the cells
showed cytoplasmic protrusions, which were even
more pronounced after maturation on day 7 (fig. 3A).
Moreover, size and granularity of the cells increased
during differentiation and maturation, which is reflected
in increased forward and side scatter signals in the flow
Mo-DC viability and yield (%)
Absolute cell count
100 1.5×10
75
1.0×10
50
25
5.0×10
0 0
Mo-DC
viability
Absolute
Mo-DC count
Mo-DC
yield
Generation of Mo-DCs | June 2016 3/8 Copyright © 2016 Miltenyi Biotec GmbH. All rights reserved.
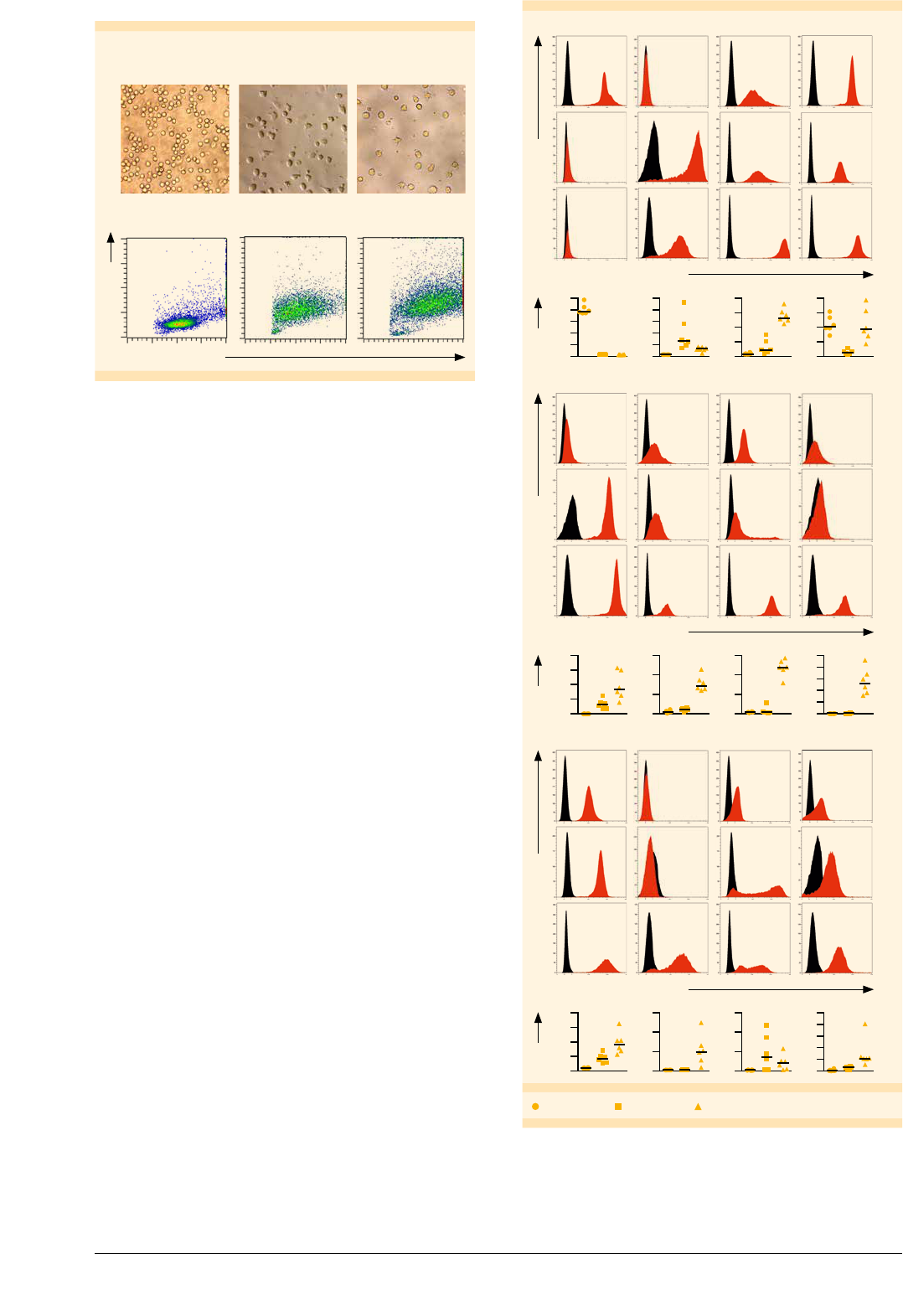
cytometry analysis (fig. 3B).
A
Monocytes
(day 0)
Immature Mo-DCs
(day 6)
Mature Mo-DCs
(day 7)
Forward scatter
Side scatter
B
Figure : Morphology of monocytes, imMo-DCs, and mMo-DCs.
Cells were analyzed on days , , and of differentiation/maturation.
(A) Images were captured on a light microscope using phase contrast
at a × magnification. (B) Assessment of relative cell size and
granularity by flow cytometry using forward and side scatter channels,
respectively. , events are shown for each dot plot.
Mature Mo-DCs express characteristic
DC surface markers
Flow cytometry analysis of cell surface markers showed
that in vitro generated Mo-DCs assumed the typical DC
phenotype (fig. 4). During differentiation monocytes
down-regulated the expression of CD14. In addition,
mMo-DCs expressed various DC markers that are involved
in the formation of immunological synapse between DC
and naive T cells, including the costimulatory proteins
CD80 and CD86, the cell adhesion molecule CD54, and
antigen-presenting molecules MHC I (HLA-ABC) and MHC II
(HLA-DR). Mature Mo-DCs also expressed the DC activation
markers, CD83, CD25, and CD40, which were up-regulated
accordingly. CCR7, which is required for migration of DCs
to draining lymph nodes, was also up-regulated. The
highest expression levels of the antigen uptake receptors,
CD209 and CD206, were detected on imMo-DCs (fig. 5),
corresponding to their antigen uptake function.
Fluorescence intensity
Relative cell number
MonocytesimMo-DCsmMo-DCs
HLA-ABCHLA-DR
CD209CD14
100
80
60
40
20
0
250
200
150
100
50
0
800
600
400
200
0
200
150
100
50
0
MFI
Fluorescence intensity
MonocytesimMo-DCsmMo-DCs
CD83CD86CD80CD40
100
80
60
40
20
0
15 150
10 100
5 50
0 0
400
300
200
100
0
Relative cell number
MFI
Fluorescence intensity
MonocytesimMo-DCsmMo-DCs
CCR7CD1aCD25CD54
100
80
60
40
20
0
15 150
10 100
5 50
0 0
400
300
200
100
0
Relative cell numberMFI
Monocytes imMo-DCs mMo-DCs
Figure : Immunophenotyping of monocytes, imMo-DCs, and
mMo-DCs.
Cells were labeled on days , , and with antibodies specific
for the respective markers and analyzed by flow cytometry. The histograms
show the results from one representative experiment Markerspecic
and isotype control labeling are shown in red and black respectively
The plots below the histograms depict the MFI for each marker on
monocytes imMODCs and mMoDCs in six independent experiments
Generation of Mo-DCs | June 2016 4/8 Copyright © 2016 Miltenyi Biotec GmbH. All rights reserved.
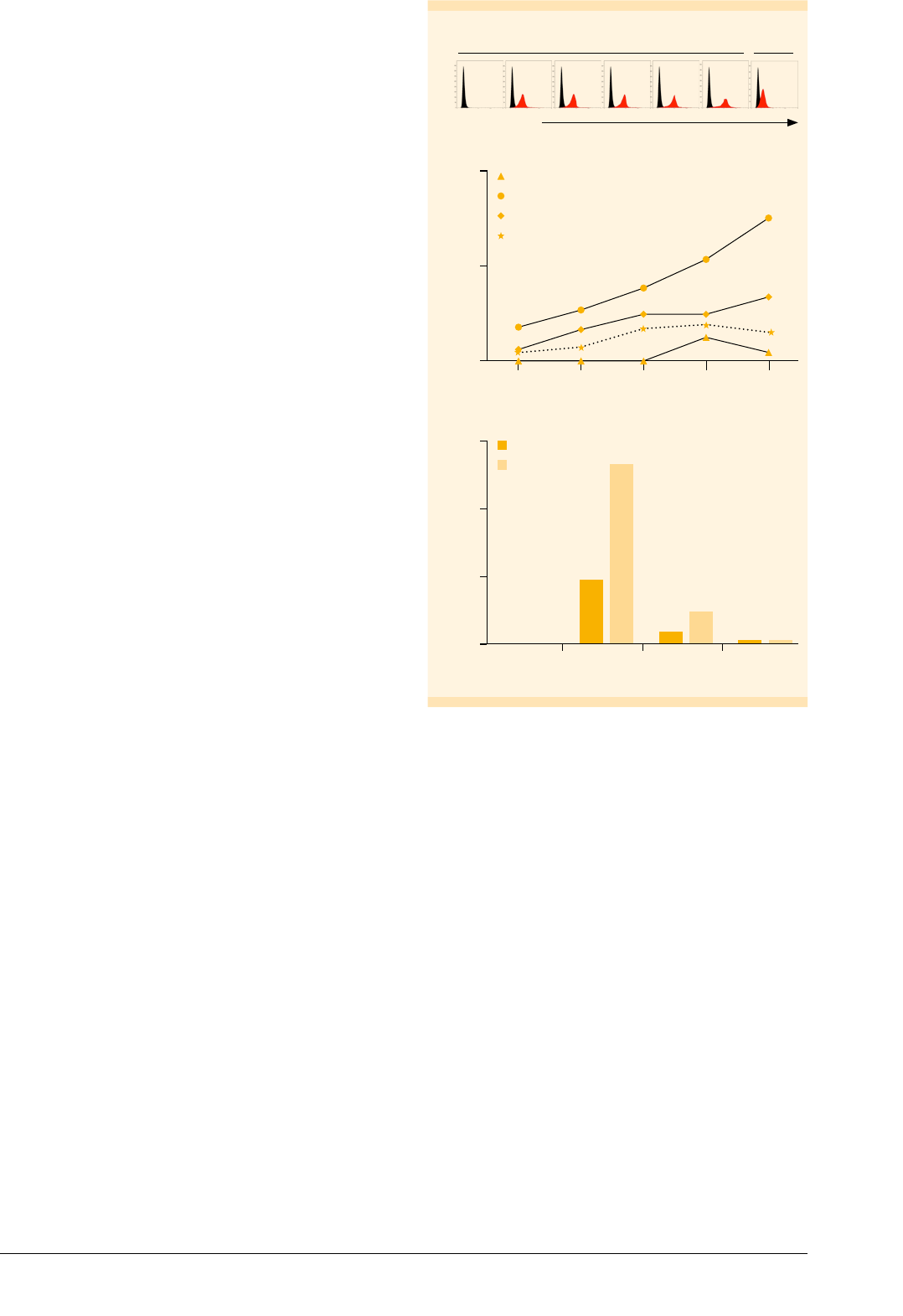
Immature Mo-DCs have the highest antigen
uptake capacity
Antigen uptake by antigen-presenting cells can occur
specifically via receptor-mediated endocytosis or non-
specifically via pinocytosis or phagocytosis. To assess
the pinocytosis capacity of in vitro generated Mo-DCs in
comparison to monocytes, cells were cultured at 37 °C for
up to 1 h in the presence of FITC-dextran. Non-specific
binding of FITC-dextran to the cell surface was determined
by incubating samples on ice throughout the procedure.
The MFI of FITC was analyzed by flow cytometry after
various time points (fig. 5A). Subtraction of MFI
Ice
(samples
on ice) from MFI (samples at 37 °C) allowed for assessment
of the relative antigen uptake capacities (fig. 5B). Immature
Mo-DCs had the highest antigen uptake capacity, reflected
in a continuous increase in MFI over the entire time course.
Mature Mo-DCs from day 7 showed decreased antigen
uptake activity compared to imMo-DCs throughout the
time course. The antigen uptake was further decreased
when Mo-DCs were matured for additional 2 days (day 9).
Monocytes showed a negligible antigen uptake capacity
compared to Mo-DCs.
Additionally, flow cytometry analysis showed that
imMo-DCs exhibited the highest expression of CD206 and
CD209 (fig. 5C). These results correlate with the finding that
imMo-DCs lose their antigen uptake function upon maturation.
FITC-dextran
Rel. cell number
A
37 °C Ice
0 min 5 min 10 min 20 min 30 min 60 min 60 min
MFI – MFI
Ice
15
10
5
0
Monocytes
imMo-DCs
mMo-DCs (day 7)
mMo-DCs (day 9)
B
105 20 30 60
Time (min)
MFI
150
100
50
0
Monocytes mMo-DCs
(day 9)
mMo-DCs
(day 7)
imMo-DCs
CD206
CD209
C
Figure : Antigen uptake capacity of monocytes and Mo-DCs.
(A) Flow cytometry results from a representative experiment with
imMo-DCs cultured at °C in the presence (red) or absence (black)
of FITC-dextran for the indicated amounts of time. Non-specific binding
was determined by incubating cells on ice. (B) Relative FITC-dextran
uptake was calculated by subtracting the MFI of cells incubated for
minutes on ice (MFI
Ice
) from the MFI of cells incubated at °C (MFI).
Results are shown for monocytes, imMo-DCs, and mMo-DCs (days
and ). (C) Monocytes and Mo-DCs were labeled with CD-APC or
CD-APC antibodies and analyzed by flow cytometry.
Mature Mo-DCs migrate towards CCL19
Upon activation of DCs in the periphery, the cells migrate
to the draining lymph nodes where they encounter naive
T cells. Migration depends on the expression of CCR7
on the DC surface
11
. The CCR7 ligand CCL19 acts as a
chemoattractant for DCs and is expressed in the lymph
node areas characterized by high T cell densities. Figure 4
indicates that CCR7 expression was up-regulated during
Mo-DC maturation. To measure the migration capacity of
in vitro generated mMo-DCs, we used Transwell Plates.
Figure 6 shows that mMo-DCs migrated towards a CCL19
stimulus in a dose-dependent manner.
Generation of Mo-DCs | June 2016 5/8 Copyright © 2016 Miltenyi Biotec GmbH. All rights reserved.
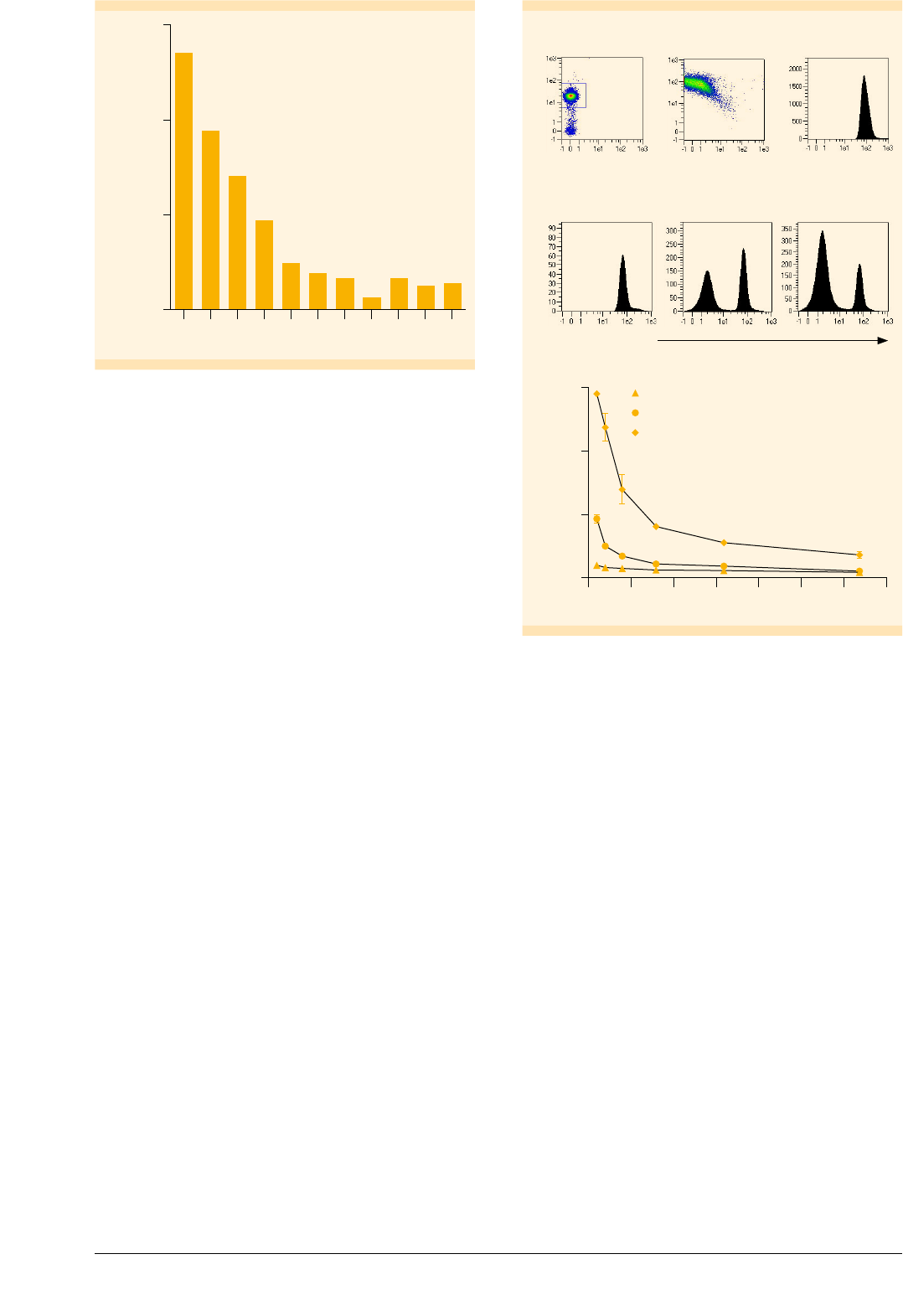
Number of cells that migrated
CCL19 concentration (ng/mL)
60,000
40,000
20,000
0
200 100 50 25
12.5 6.25 3.125 1.56 0.78 0.39
0
Figure : Migration of mMo-DCs towards a CCL stimulus. The
mMo-DCs (× cells) were placed in the upper compartment of a
-well Transwell Plate. Media containing different concentrations
of CCL were placed in the lower compartment. After h the total
number of cells that migrated to the lower compartment was
determined by flow cytometry.
Mature Mo-DCs have the capacity to prime naive T cells
After migration to the lymph nodes, DCs can induce the
proliferation of naive T cells. We tested the capacity of
monocytes and Mo-DCs to induce T cell proliferation in
an MLR. To this end, we isolated allogeneic naive
CD45RA
+
CD45RO
–
T cells to high purities by MACS®
Technology (fig. 7A) and labeled their plasma membrane
with CellTrace Violet (fig. 7B). After coculturing the labeled
T cells with monocytes or Mo-DCs for 7 d, we determined
the cell numbers of T cells and analyzed the CellTrace Violet
staining by flow cytometry. As with each division of the
Tcells the dye gets more diluted in the plasma membrane,
an increase in unlabeled cells indicates a high T cell
proliferation rate. In contrast to monocytes both imMo-DCs
and mMo-DCs increased the number of CellTrace Violet–
negative T cells in the culture (fig. 7C). This correlated with
higher T cell numbers, as determined by cell counting.
Tcell proliferation was highest when mMo-DCs were used
as antigen-presenting cells (fig. 7D), which is in line with
the aforementioned result (fig. 4A) showing that receptors
involved in T cell priming are up-regulated on Mo-DCs
upon maturation.
Rel. cell number
CellTrace Violet
CD45RA
CD45RO
Enriched naive T cells
CD4
R1
96%
A B
CellTrace Violet
Rel. cell number
C
Monocytes imMo-DCs mMo-DCs
Number of T cells
3×10
2×10
1×10
0
Monocytes
imMo-DCs
mMo-DCs
D
20100 4030 6050 70
T cell: Mo-DC ratio
Figure : Induction of T cell proliferation by Mo-DCs. (A) Naive
CD
+
T Cells were isolated from PBMCs by MACS Technology. Isolated
cells were stained with CD-PE or CDRA-PE/CDRO-APC antibodies
and analyzed by flow cytometry using the MACSQuant Analyzer
. (B) Isolated naive T cells were labeled with CellTrace Violet and
analyzed by flow cytometry. (C) CellTrace Violet–labeled naive T cells
were cocultured with monocytes, imMo-DCs, and mMo-DCs at a ratio
of : for d and analyzed by flow cytometry. (D) Naive T cells were
cocultured with imMo-DCs and mMo-DCs at various ratios. After d
the numbers of T cells were determined by flow cytometry based
on scatter signals. Dead cells were excluded from the analysis by PI
fluorescence. One of three representative experiments is shown.
Mature Mo-DCs secrete IL-12 upon stimulation
with soluble CD40L
In general, DCs have the capacity to secrete both
pro-inflammatory and immunoregulatory cytokines,
depending on the stimulus received. T cell–mediated
CD40L stimulation of Mo-DCs induces the production
of T
h1-polarizing IL-12, but also the secretion of the
immunosuppressive IL-10
12-14
. However, for studies towards
the development of cancer therapies it is desirable to
generate Mo-DC populations secreting high amounts of
IL-12 and low amounts of IL-10 as these cells generate a more
effective antitumor response via induction of T
h1 cells.
Generation of Mo-DCs | June 2016 6/8 Copyright © 2016 Miltenyi Biotec GmbH. All rights reserved.
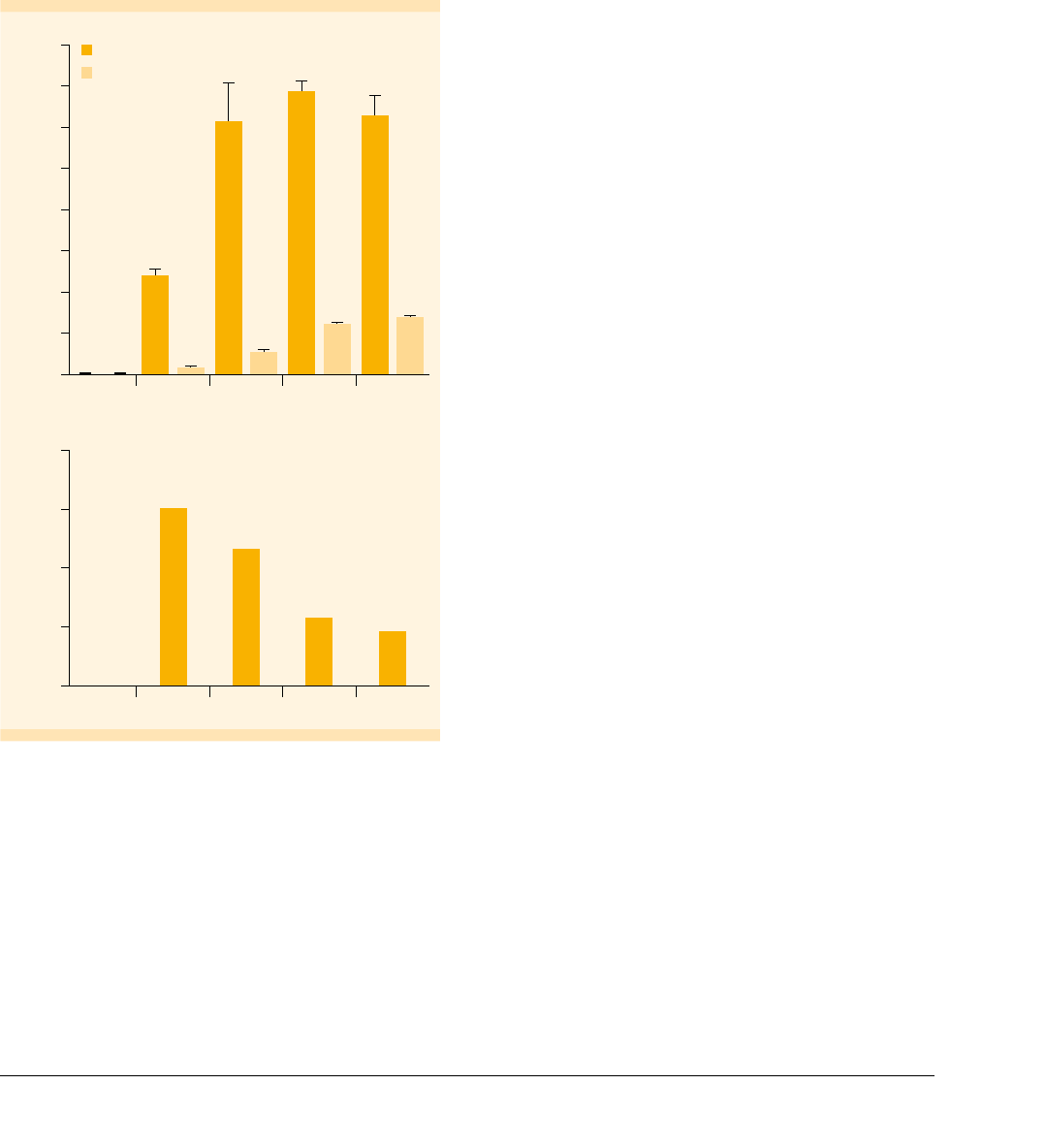
To determine the capacity of in vitro generated Mo-DCs
to secrete IL-12 and IL-10, we stimulated mMo-DCs under
various conditions for one day: i) incubation with soluble
Human CD40-Ligand (Miltenyi Biotec), which forms
multimers in vitro, and ii) coculture with a J558L cell line
expressing CD40L. Stimulation with soluble CD40L led to
production of high levels of IL-12 and low levels of IL-10.
Co-culture with CD40L-expressing J588L cells resulted in an
overall increase in secretion of both IL-12 and IL-10 (fig. 8A).
However, the ratio of IL12/IL-10 was higher after stimulation
with soluble CD40L than after coculture with the cell line
(fig. 8B). Thus, recombinant human CD40L multimers
represent an attractive alternative to CD40L-transfected
cell lines, allowing for CD40 stimulation of Mo-DCs under
defined conditions.
Cytokine concentration (pg/mL)
800
700
500
300
100
600
400
200
0
Control J558
CD40L***
Soluble
CD40L
J558
CD40L*
J558
CD40L**
IL-12p70
IL-10
A
Ratio IL-12p70:IL-10
20
15
10
5
0
Soluble
CD40L
J558
CD40L***
J558
CD40L**
J558
CD40L*
B
Figure : IL- and IL- secretion by mMo-DCs upon stimulation
with CDL. (A) The mMo-DCs were cultured for h in the absence
or presence of soluble CDL ( µg/mL) or a J cell line expressing
CDL (*× cells/well, **× cells/well, ***× cells/well).
IL-p and IL- concentrations in the culture supernatant were
determined by ELISA. (B) The ratios of IL-p vs. IL- were calculated
based on the results shown in (A).
Conclusion
• This applications note describes procedures that cover
a complete workflow for the generation and phenotypic
and functional analysis of mMo-DCs.
• CD14 MicroBeads enable the isolation of viable CD14
+
monocytes to high purities, with high yields. The
isolated monocytes can be easily differentiated into
Mo-DCs.
• A straightforward and reliable differentiation/
maturation protocol based on cytokines from Miltenyi
Biotec enables the effective generation of mMo-DCs.
• In vitro generated imMo-DCs and mMo-DCs possess the
characteristics of DCs in terms of i) morphology, ii)
surface marker expression, iii) antigen uptake capacity,
iv) migration towards a CCL19 stimulus, v) induction of
Tcell proliferation, and vi) capacity to secrete IL-12 in
response to CD40L stimulation.
• Comprehensive phenotypic analysis is accomplished
using flow cytometry tools from Miltenyi Biotec,
including the powerful MACSQuant Analyzer 10 and
a wide range of MACS Antibodies.
• Recombinant human CCL19 from Miltenyi Biotec is a
potent chemoattractant for CCR7-expressing Mo-DCs.
• CD40L from Miltenyi Biotec effectively stimulates
Mo-DCs to secrete IL-12. The resulting ratio of IL-12:IL-10
is at least as high as with a CD40L-expressing cell line.
Generation of Mo-DCs | June 2016 7/8 Copyright © 2016 Miltenyi Biotec GmbH. All rights reserved.
Product Order no.
Cell isolation
CD14 MicroBeads, human 130-050-201
CD MicroBeads, human – lyophilized --
Naive CD
+
T cell Isolation Kit II, human --
Flow cytometry
MACSQuant Analyzer --
MACSQuantify Software --
Anti-HLA-DR-FITC, human *
Anti-HLA-ABC-FITC, human *
CDa-PE, human *
CD-PE, human *
CD-FITC, human *
CD-APC, human *
CD-APC, human *
CDRA-PE, human *
CDRO-APC, human *
CD-PE, human *
CD-PE, human *
CD-APC, human *
CD-PE, human *
CD (CCR)-APC, human *
CD-APC, human *
CD (DC-SIGN)-APC, human *
Propidium Iodide Solution --
Cell culture
Human CCL (MIP-β), research grade --**
Human CD-Ligand, premium grade --**
Human GM-CSF, premium grade --**
Human IL-β, premium grade --**
Human IL-, premium grade --**
Human IL-, premium grade --**
Human TNF-α, premium grade --**
* for different package sizes and additional conjugates, visit
www.miltenyibiotec.com/antibodies
** Order numbers are provided for µg sizes.
For different quality grades and additional package sizes, visit
www.miltenyibiotec.com/cytokines
References
1. Palucka, K. and Banchereau, J. (2012) Cancer immunotherapy via
dendritic cells. Nat. Rev. Cancer 12: 265–277.
2. Banchereau, J. and Steinman, R.M. (1998) Dendritic cells and the control
of immunity. Nature 392: 245–252.
3. Acuto, O. and Michel, F. (2003) CD28-mediated co-stimulation: a
quantitative support for TCR signalling. Nat. Rev. Immunol. 3: 939–951.
4. van Kooten, C. and Banchereau, J. (2000) CD40-CD40 ligand. J. Leukoc.
Biol. 67: 2–17.
5. Figdor, C.G. et al. (2004) Dendritic cell immunotherapy: mapping the
way. Nat. Med. 10: 475–480.
6. Hubo, M. et al. (2013) Costimulatory molecules on immunogenic versus
tolerogenic human dendritic cells. Front. Immunol. 4: 51–63.
7. Romani, N. et al. (1994) Proliferating dendritic cell progenitors in human
blood. J. Exp. Med. 180: 83–93.
8. Caux, C. et al. (1994) Activation of human dendritic cells
through CD40 cross-linking. J. Exp. Med. 180: 1263–1272.
9. Jonuleit, H. et al. (1997) Pro-inflammatory cytokines and prostaglandins
induce maturation of potent immunostimulatory dendritic cells under
fetal calf serum-free conditions. Eur. J. Immunol. 27: 3135–3142.
10. Feuerstein, B. et al. (2000) A method for the production of
cryopreserved aliquots of antigen-preloaded, mature dendritic cells
ready for clinical use. J. Immunol. Methods. 245: 15–29.
11. Martín-Fontecha, A. et al. (2003) Regulation of dendritic cell migration
to the draining lymph node: impact on Tlymphocyte traffic and
priming. J. Exp. Med. 198: 615–621.
12. Moser, M. and Murphy, K.M. (2000). Dendritic cell regulation of TH1-TH2
development. Nat. Immunol. 1: 199–205.
13. Trinchieri, G. (1994) Interleukin-12: a cytokine produced by antigen-
presenting cells with immunoregulatory functions in the generation of
T-helper cells type 1 and cytotoxic lymphocytes. Blood. 84: 4008–4027.
14. Iwasaki, A. and Kelsall, B.L. (1999) Freshly isolated Peyer’s patch, but
not spleen, dendritic cells produce interleukin 10 and induce the
differentiation of T helper type 2 cells. J. Exp. Med. 190: 229–239.
miltenyibiotec.com
Miltenyi Biotec GmbH | Friedrich-Ebert-Straße 68 | 51429 Bergisch Gladbach | Germany | Phone +49 2204 8306-0 | Fax +49 2204 85197
macs@miltenyibiotec.de | www.miltenyibiotec.com
Miltenyi Biotec provides products and services worldwide. Visit www.miltenyibiotec.com/local to nd your nearest Miltenyi Biotec contact.
Unless otherwise specically indicated, Miltenyi Biotec products and services are for research use only and not for therapeutic or diagnostic use. MACS,
the MACS logo, MACSQuant, and MACSQuantify are registered trademarks or trademarks of Miltenyi Biotec GmbH. All other trademarks mentioned in this
document are the property of their respective owners and are used for identication purposes only. Copyright © 2016 Miltenyi Biotec GmbH. All rights reserved.
Generation of Mo-DCs | June 2016 8/8 Copyright © 2016 Miltenyi Biotec GmbH. All rights reserved.
